Long-Term Safety and Effectiveness of Dimethyl Fumarate in Patients with Multiple Sclerosis Treated in Routine Medical Practice: Final Analysis of the ESTEEM Study
- PMID: 39673658
- PMCID: PMC11762038
- DOI: 10.1007/s40120-024-00680-z
Long-Term Safety and Effectiveness of Dimethyl Fumarate in Patients with Multiple Sclerosis Treated in Routine Medical Practice: Final Analysis of the ESTEEM Study
Abstract
Introduction: Dimethyl fumarate (DMF) has demonstrated a favorable benefit-risk profile in patients with relapsing-remitting multiple sclerosis (RRMS) in clinical and real-world studies. The ESTEEM study (NCT02047097) was conducted to assess the long-term safety and effectiveness of delayed-release DMF in patients with relapsing forms of MS in routine clinical practice. We report final outcomes from ESTEEM with up to 6.5 years of follow-up.
Methods: Patients newly prescribed DMF were recruited from 393 sites globally. The primary objective was to assess the incidence and type of serious adverse events (SAEs) and adverse events (AEs) leading to discontinuation of DMF. Secondary objectives included assessment of DMF effectiveness on annualized relapse rate (ARR) and patient-reported outcomes (PROs).
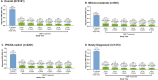
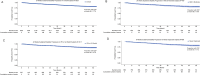
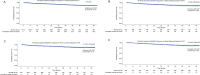
Results: Overall, 5124 patients received ≥ 1 dose of DMF. The mean (standard deviation [SD]) age at enrollment was 40.0 (11.2) years; 74% of patients were female. Patients received DMF for a mean (SD) duration of 31.0 (22.7) months. Primary reasons for discontinuation were AEs (n = 1237; 24%); the most common were gastrointestinal AEs (n = 469; 9%), blood and lymphatic disorders (n = 218; 4%), and vascular disorders (n = 200; 4%). SAEs occurred in 391 (8%) patients, most commonly infections and infestations (n = 102; 2%). Adjusted ARR declined by 90% (95% confidence interval [CI]: 90-91%; p < 0.0001), from 0.81 (95% CI 0.79-0.84) 12 months before enrollment to 0.08 (95% CI 0.08-0.09) 6 years after enrollment. The estimated proportion of patients free from confirmed disability progression sustained over 48 weeks was 87.0% at month 60. Mean scores for physical and psychological impact, fatigue, health, and productivity remained stable over 5 years.
Conclusion: DMF demonstrated a safety profile in real-world clinical practice consistent with the known profile of DMF. Relapse rates were low and both ARR and PROs remained stable over time.
Trial registration: ClinicalTrials.gov Identifier NCT02047097.
Keywords: Dimethyl fumarate; Disease-modifying treatment; Effectiveness; Multiple sclerosis; Real world.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Krupa Shah Pandey: consulting fees from Bristol Myers Squibb; fees for non-CME services and/or speaker bureaus from Alexion, Biogen, Bristol Myers Squibb, Genentech, and Sanofi; research support from Genentech. Kathryn Giles: consulting fees from Biogen, EMD Serono, and Genzyme. Konstantin Balashov: consulting fees from Genentech; research support from Biogen. Richard Macdonell: consulting fees and research support from Biogen, Celgene, Genentech, Genzyme, and Teva; speaker bureau for Biogen, Celgene, Genentech, Genzyme, and Teva. Jörg Windsheimer: consulting fees from Genzyme, Merck, Novartis, Roche, and Teva. Mikel Martinez: consulting fees from Genzyme, Novartis, and Pfizer. Ivan Božin, Matthew Scaramozza, Oksana Mokliatchouk, Zhaonan Sun, Nicholas Belviso, Yayoi Sato, Xiaochen Lin: employees of Biogen and may hold stock in the company. Stephanie Raynaud: employee of and held stock/stock options in Biogen at the time this work was conducted. Annette Okai: consulting fees from Biogen, Bristol Myers Squibb, EMD Serono, Novartis, Roche-Genentech, and Sanofi-Genzyme; research support from Biogen, Bristol Myers Squibb, Roche-Genentech, and Sanofi Genzyme; speaker bureau for Biogen, Bristol Myers Squibb, EMD Serono, Roche-Genentech, and Sanofi-Genzyme. Ethical Approval: The study was conducted in accordance with protocol, the International Council for Harmonization Guideline E6, all applicable local regulatory requirements, and ethical principles based on the Declaration of Helsinki.
Figures


References
-
- National MS Society. Disease-modifying therapies for MS. 2021 [cited 29 Feb 2024]; Available from: https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFile...
-
- Mokry C, Warnke C, Gehring K, Hegen H, Salmen A, Kraemer M, et al. Implementation study of the 2021 German guideline for diagnosis and treatment of multiple sclerosis. Mult Scler Relat Disord. 2022;57: 103434. - PubMed
-
- Biogen. Tecfidera Prescribing Information. 2023 [cited 2022 15 Aug]; Available from: https://www.tecfidera.com/content/dam/commercial/multiple-sclerosis/tecf...
-
- Biogen. Tecfidera Summary of Product Characteristics. 2022 [cited 2024 22 March]; Available from: https://www.ema.europa.eu/en/documents/product-information/tecfidera-epa...
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

