Excess shed mesothelin disrupts pancreatic cancer cell clustering to impair peritoneal colonization
- PMID: 39673668
- PMCID: PMC11646052
- DOI: 10.1096/fj.202400446R
Excess shed mesothelin disrupts pancreatic cancer cell clustering to impair peritoneal colonization
Abstract
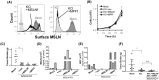
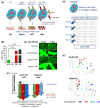
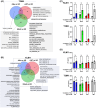
Peritoneum is the second most common site of metastasis in patients with pancreatic ductal adenocarcinoma (PDAC). Peritoneal colonization is impaired in PDAC cells with knockout (KO) of the cancer surface antigen mesothelin (MSLN) or by introducing Y318A mutation in MSLN to prevent binding to mucin-16 (MUC-16). MSLN has a membrane-bound form but is also shed to release soluble MSLN (sMSLN). Their individual roles in peritoneal metastasis are unknown. Here, a C-terminal truncated MSLN mutant (∆591) incapable of cell membrane insertion but proficient in secretion was engineered. Expression of ∆591 MSLN failed to rescue peritoneal metastasis in MSLN KO cells and inhibited peritoneal colonization when overexpressed in WT PDAC cells. Exposing PDAC cells to conditioned medium (CM) containing excess sMSLN impaired cancer cell clustering in vitro and in peritoneal fluid in vivo, while CM containing only Y318A sMSLN did not. These data demonstrate that interaction of membrane-bound MSLN with MUC-16 promotes cell clustering that is critical for efficient peritoneal metastasis. However, peritoneal colonization by MSLN KO cells was rescued by expression of ∆591 mutant MSLN bearing Y318A mutation, suggesting that sMSLN also has a MUC-16-independent role in peritoneal spread. Alterations in inflammatory signaling pathways occurred following KO cell exposure to CM containing sMSLN, and CM from cancer cells with intact peritoneal metastasis provoked increased KO cell secretion of IL-1α. While excess sMSLN inhibits cell clustering and peritoneal colonization, sMSLN may also promote PDAC peritoneal metastasis independent of MUC-16.
Keywords: IL‐1α; cell clustering; mesothelin; pancreatic cancer; peritoneal metastasis.
Published 2024. This article is a U.S. Government work and is in the public domain in the USA. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures



References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17‐48. - PubMed
-
- Einama T, Kamachi H, Nishihara H, et al. Co‐expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas. 2011;40:1276‐1282. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

