Open Label Vancomycin in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Improved Colonic Disease Activity and Associations With Changes in Host-Microbiome-Metabolomic Signatures
- PMID: 39673746
- PMCID: PMC11831226
- DOI: 10.1093/ecco-jcc/jjae189
Open Label Vancomycin in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Improved Colonic Disease Activity and Associations With Changes in Host-Microbiome-Metabolomic Signatures
Abstract
Background: We conducted a single-arm interventional study, to explore mucosal changes associated with clinical remission under oral vancomycin (OV) treatment, in primary sclerosing cholangitis-associated inflammatory bowel disease (PSC-IBD); NCT05376228.
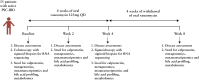
Methods: Fifteen patients with PSC and active colitis (median fecal calprotectin 459 µg/g; median total Mayo score 5) were treated with OV (125 mg QID) for 4 weeks and followed-up for a further 4 weeks of treatment withdrawal (8 weeks, end-of-study). Colonic biopsies were obtained at baseline and Week 4. Clinical assessments, and serum and stool samples (metagenomics, metatranscriptomics, and metabolomics) were collected at Weeks 0, 2, 4, and 8. The primary efficacy outcome measure was the induction of clinical remission.
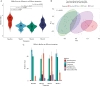
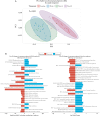
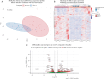
Results: Oral vancomycin resulted in clinical remission in 12/15 patients and significant reductions in fecal calprotectin. Oral vancomycin was associated with reduced abundances of Lachnospiraceae, genera Blautia and Bacteroides; and enrichment of Enterobacteriaceae, and genera Veillonella, Akkermansia, and Escherichia. Oral vancomycin treatment was associated with the downregulation of multiple metatranscriptomic pathways (including short-chain fatty acid [SCFA] metabolism and bile acid [BA] biotransformation), along with host genes and multiple pathways involved in inflammatory responses and antimicrobial defence; and an upregulation of genes associated with extracellular matrix repair. Oral vancomycin use resulted in the loss of specific fecal SCFAs and secondary BAs, including lithocholic acid derivatives. Colitis activity relapsed following OV withdrawal, with host mucosal and microbial changes trending toward baseline.
Conclusions: Four weeks of OV induces remission in PSC-IBD activity, associated with a reduction in gut bacterial diversity and compositional changes relating to BA and SCFA homeostasis.
Keywords: Primary sclerosing cholangitis; inflammatory bowel disease; vancomycin.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
MNQ has received speaker fees from Pfizer, Janssen, Takeda, Tillotts, Falk, and Nordic Pharma and consultancy fees from Nordic Pharma. BM has no conflicts of interest to declare. JRM has received consultancy fees from Cultech Ltd. and EnteroBiotix Ltd. PJT receives institutional funding from the NIHR Birmingham BRC. This article presents independent research supported by the Birmingham NIHR BRC, based at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. PJT has received advisory board/consultancy fees from Chemomab, Cymabay, Albireo/Ipsen Pharma, Advanz Pharma/Intercept, Dr. Falk Pharma, GlaxoSmithKline, Gilead Sciences, Mirum, and Pliant Pharma. PJT has received independent grant funding from Albireo/Ipsen, Advanz Pharma/Intercept, Bristol Myers Squibb, GlaxoSmithKline, Gilead Sciences, Guts UK, the European Association for Study of the Liver (EASL), Innovate UK, LifeArc, the Medical Research Foundation, the NIHR, Mirum, PSC Support, Regeneron, and the Wellcome Trust.
Figures




References
-
- Karlsen TH, Folseraas T, Thorburn D, Vesterhus M.. Primary sclerosing cholangitis—a comprehensive review. J Hepatol 2017;67:1298–323. - PubMed
-
- Culver EL, Bungay HK, Betts M, et al. Prevalence and long-term outcome of sub-clinical primary sclerosing cholangitis in patients with ulcerative colitis. Liver Int 2020;40:2744–57. - PubMed
-
- Trivedi PJ, Hirschfield GM.. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut 2021;70:1989–2003. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

