Safety and quality of life with maintenance olaparib plus bevacizumab in older patients with ovarian cancer: subgroup analysis of PAOLA‑1/ENGOT-ov25
- PMID: 39673779
- PMCID: PMC12311279
- DOI: 10.1093/oncolo/oyae322
Safety and quality of life with maintenance olaparib plus bevacizumab in older patients with ovarian cancer: subgroup analysis of PAOLA‑1/ENGOT-ov25
Abstract
Background: In PAOLA-1/ENGOT-ov25, the addition of olaparib to bevacizumab maintenance improved overall survival in patients with newly diagnosed advanced ovarian cancer. We describe the safety profile and quality of life (QoL) of this combination in older patients in PAOLA-1.
Methods: Safety (CTCAE v4.03) and QoL (EORTC QoL Questionnaires Core 30 and Ovarian 28) data were collected. We compared safety by age (≥70 vs <70 years) in the olaparib-containing arm. QoL by treatment arm was assessed in older patients. Geriatric features, including Geriatric Vulnerability Score (GVS), were also gathered.
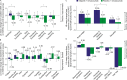
Results: Of 806 patients randomized, 142 were ≥70 years old (olaparib-containing arm: n = 104; placebo arm: n = 38). Older patients treated with olaparib exhibited a similar safety profile to younger patients, except for higher rates of all grades of lymphopenia and grade ≥3 hypertension (31.7% vs 21.6%, P =.032 and 26.9% vs 16.7%, P =.019, respectively). No hematological malignancy was reported. Two years after randomization, mean Global Health Status and cognitive functioning seemed better with olaparib than bevacizumab alone (adjusted mean difference: +4.47 points [95% CI, -0.49 to 9.42] and +4.82 [-0.57 to 10.21], respectively), and other QoL items were similar between arms. In the olaparib-containing arm, older patients with baseline GVS ≥ 1 (n = 48) exhibited increased toxicity and poorer QoL than those with GVS of 0 (n = 34).
Conclusion: Among older patients in PAOLA-1, olaparib plus bevacizumab had a manageable safety profile and no adverse impact on QoL. Additional data are required to confirm these results in more vulnerable patients.(ClinicalTrials.gov Identifier: NCT02477644).
Keywords: elderly; geriatric assessment; ovarian cancer; quality of life; safety.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
C.M.: reports no conflict of interest.
C.F.: has received advisory or consultancy honoraria from Leo Pharma, Pfizer, MSD Oncology, Teva, AstraZeneca, Baxter, Eisai, Janssen Oncology, Novartis, Chugai Pharma, and Astellas Pharma; and non-financial support from Janssen Oncology, Pierre Fabre, AstraZeneca, and Leo Pharma.
S.C.: reports no conflict of interest.
C.C.: reports no conflict of interest.
L.M.: reports no conflict of interest.
F.R.: reports advisory boards for Eisai, Novartis, and Viatris-Mylan.
F.J.: reports consulting fees from GSK (consulting for the European Medicines Agency); payment/honoraria (advisory board) from AstraZeneca, Merck Sharp & Dohme LLC (MSD), and Seagen; payment/honoraria (advisory board, lectures) from GSK and Clovis; and support for attending meetings and/or travel (symposium) from GSK, AstraZeneca, and MSD.
M.M.: reports no conflict of interest.
A.M.M.: reports no conflict of interest.
E.M.G.-A.: reports consulting fees from AstraZeneca-MSD, Clovis Oncology, GSK-Tesaro, PharmaMar, and Roche; speaker bureau/expert testimony honorarium from AstraZeneca-MSD, PharmaMar, Roche, GSK-Tesaro, and Clovis Oncology; and travel support from Roche, GSK-Tesaro, and Baxter.
C.S.: reports no conflict of interest.
H.F.: reports no conflict of interest.
I.V.: reports consulting fees from Agenus, Akesobio, AstraZeneca, Bristol Myers Squibb, Deciphera Pharmaceuticals, Eisai, Elevar Therapeutics, F. Hoffmann-La Roche, Genmab, GSK, Immunogen, Jazz Pharma, Karyopharm, Mersana, MSD, Novocure, Novartis, Oncoinvent, OncXerna, Sanofi, Regeneron, Seagen, Sotio, Verastem Oncology, and Zentalis; contracted research (via KULeuven) from Oncoinvent AS; corporate-sponsored research from Amgen and Roche; and accommodations and travel expenses from Karyopharm, Genmab, and Novocure.
G.P.: reports no conflict of interest.
G.L.: reports no conflict of interest.
A.A.: reports having received consulting fees and travel expenses from AstraZeneca, BMS, and Roche; travel expenses from Novartis and Pfizer; and consulting fees from Amgen.
U.C.: reports advisory fees from AstraZeneca; and speakers’ bureau fees from Lilly and AstraZeneca.
F.M.: reports honoraria from AGO Research GmbH (funding indirectly provided by AstraZeneca); and personal fees from Roche, AstraZeneca, Pfizer, Tesaro, Novartis, Amgen, PharmaMar, Genomic Health, CureVac, Eisai, Clovis Oncology, Janssen-Cilag, Gilead, GSK, MSD, Seagen, Myriad Genetics, and Pierre Fabre.
E.P.-L.: reports lecture fees, fees for serving on a speakers’ bureau, and travel support from AstraZeneca, GSK, Roche, and Tesaro; lecture fees from Clovis Oncology and Pfizer; expert testimony fees from AstraZeneca; support for attending meetings and/or travel from AstraZeneca and GSK; fees from AstraZeneca, Incyte, and Roche for participating on a data safety monitoring board or advisory board; and employment by ARCAGY Research.
I.R.-C.: reports honoraria (self) from AbbVie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro, and Clovis; honoraria (institution) from GSK, MSD, Roche, and BMS; advisory/consulting fees from AbbVie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche/Genentech, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro, and Clovis; and travel support from Roche, AstraZeneca, and GSK.
R.S.: reports advisory board for Roche, GSK, and Novartis; and non-financial support (travel, accommodation, and meeting registration fees) from Pfizer, Roche, GSK, BMS, and AstraZeneca.
Figures


References
-
- Tew WP. Ovarian cancer in the older woman. J Geriatr Oncol. 2016;7:354-361. https://doi.org/ 10.1016/j.jgo.2016.07.008 - DOI - PubMed
-
- Wright JD, Ananth CV, Tsui J, et al. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer. 2014;120:1246-1254. https://doi.org/ 10.1002/cncr.28508 - DOI - PMC - PubMed
-
- Hilpert F, du Bois A, Greimel ER, et al. Feasibility, toxicity and quality of life of first-line chemotherapy with platinum/paclitaxel in elderly patients aged >or=70 years with advanced ovarian cancer--a study by the AGO OVAR Germany. Ann Oncol. 2007;18:282-287. https://doi.org/ 10.1093/annonc/mdl401 - DOI - PubMed
-
- Schuurman MS, Kruitwagen R, Portielje JEA, et al. Treatment and outcome of elderly patients with advanced stage ovarian cancer: a nationwide analysis. Gynecol Oncol. 2018;149:270-274. https://doi.org/ 10.1016/j.ygyno.2018.02.017 - DOI - PubMed
-
- Trédan O, Geay JF, Touzet S, et al. ; Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens. Carboplatin/cyclophosphamide or carboplatin/paclitaxel in elderly patients with advanced ovarian cancer? Analysis of two consecutive trials from the Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. Ann Oncol. 2007;18:256-262. https://doi.org/ 10.1093/annonc/mdl400 - DOI - PubMed

