Improving real-world evaluation of patient- and physician-reported tolerability: niraparib for recurrent ovarian cancer (NiQoLe)
- PMID: 39673810
- PMCID: PMC11878563
- DOI: 10.1093/jncics/pkae114
Improving real-world evaluation of patient- and physician-reported tolerability: niraparib for recurrent ovarian cancer (NiQoLe)
Abstract
Background: Maintenance niraparib at an individualized starting dose (ISD) is established in platinum-sensitive recurrent ovarian cancer (PSROC). However, patients' perspectives on the burden of prolonged maintenance therapy have not been reported in prospective trials or routine practice.
Methods: In the real-life multicenter NiQoLe study, patients with PSROC received ISD maintenance niraparib. The primary objective was to describe physician-reported adverse events (AEs) leading to treatment modification during the first 3 months. Secondary endpoints included patient-reported outcomes (symptomatic AEs using PRO-CTCAE, self-reported fatigue, and impact on daily activities/function using FACT-F) collected remotely weekly using a specifically designed electronic device.
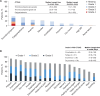
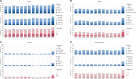
Results: Most (80%) of 139 treated patients (median age = 70 years) began niraparib at 200 mg/day. Median treatment duration was 5.7 (range = 0.2-21.4) months. During the first 3 months, 86 patients (62%) required treatment modification (median = 27 days to modification). Physician-reported grade ≥3 niraparib-related AEs occurred in 34 patients (24%); 68 patients (49%) had treatment modification for AEs, predominantly thrombocytopenia. The most frequent patient-reported AEs (PRO-CTCAE) were fatigue, insomnia, constipation, and dry mouth. Self-reported AEs were severe in 66% of patients. At baseline, 33% of patients reported severe fatigue (FACT-F), which generally persisted during niraparib. Physicians systematically underestimated major patient-reported symptoms.
Conclusions: In routine practice, niraparib dose modification was often required during the first 3 months despite individualized dosing. Physicians underestimated the burden of fatigue and symptomatic AEs. Digital self-reporting of AEs is feasible, provides patient-centered information complementing physician-reported AEs, and allows fuller appreciation of toxicity in real-world studies.
Clinical trial information: NCT03752216.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Florence Joly reports honoraria for lectures, educational events, and an expert board from GSK, honoraria for lectures and expert boards from AstraZeneca, Clovis, and MSD, honoraria for expert boards from Seagen and Novocure, support for attending meetings and/or travel from GSK, MSD, and Eisai, and financial support for national academic GINECO trials from GSK and AstraZeneca. Fernando Bazan reports payments for expert testimony from Daiichi Sankyo, Pfizer, Novartis, Clovis, Exact Sciences, and AstraZeneca and meeting/travel support from Novartis, Pfizer, Daiichi Sankyo, AstraZeneca, and GSK. Delphine Garbay reports meeting support from Gilead Oncology, MSD, AstraZeneca, Lilly, and Pfizer and participation on a data safety monitoring board/advisory board for Pfizer and MSD. Philippe Follana reports honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from GSK, Novartis, Lilly, Daiichi Sankyo, and MSD and meeting/travel support from GSK, Novartis, Gilead, and Lilly. Pierre Combe reports honoraria for lectures/presentations from MSD, BMS, and Eisai and participation in advisory boards for GSK and AstraZeneca. Anne-Claire Hardy-Bessard reports meeting/travel support from GSK and advisory board participation for GSK. Frédéric Selle reports advisory board fees from AstraZeneca, GSK/Tesaro, and MSD and honoraria for invited speaker engagements from AstraZeneca, GSK/Tesaro, MSD, and Eisai. Julien Grenier reports honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Daiichi Sankyo and Gilead and meeting/travel support from Daiichi Sankyo, Gilead, Eisai, and Lilly. Coriolan Lebreton reports honoraria for lectures/presentations from MSD, GSK, Eisai, and Clovis Oncology, support for attending meetings/travel from MSD and GSK, and participation on a data safety monitoring board/advisory board for GSK. Elise Bonnet reports grants/contracts from Gilead for a training course for employees, support to attend congresses from Lilly and Pfizer, and participation on an expert board for Gilead. Pierre Fournel reports honoraria for speaker engagements from AstraZeneca and Sanofi, support from Takeda to attend ESMO 2022 and ESMO 2023, and participation in advisory boards for BMS and MSD. Jérôme Alexandre reports research grants to his institution from GSK, MSD, and Janssen, consulting fees from AstraZeneca, MSD, Eisai, GSK, and Pfizer, personal honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from AstraZeneca, Eisai, MSD, GSK, and Novartis, and meeting/travel support from AstraZeneca and Eisai. Delphine Le Roux reports meeting/travel support from GSK. Stanislas Quesada reports meeting/travel support and honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from GSK and AstraZeneca. Jean-Emmanuel Kurtz reports travel expenses from GSK. Yaelle Ouldbey, Élise Champeaux-Orange, Eric Legouffe, Pierre-Emmanuel Brachet, Dominique Spaeth, Olfa Derbel, Yolanda Fernandez Diez, Valérie Delecroix, Sheik Emambux, Thomas Grellety, Dominique Mille, Hubert Orfeuvre, Catherine Favier, and Marie-Ange Mouret-Reynier have no disclosures.
Figures
References
-
- Poveda A, Floquet A, Ledermann JA, et al. ; SOLO2/ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:620-631. - PubMed
-
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. ; SOLO2/ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284. - PubMed
-
- Mirza MR, Monk BJ, Herrstedt J, et al. ; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164. - PubMed
-
- Gonzalez-Martin A, Pothuri B, Vergote I, et al. ; PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
