FKBP51 overexpression in the corticolimbic system stabilizes circadian rhythms
- PMID: 39674313
- PMCID: PMC11750455
- DOI: 10.1016/j.cstres.2024.12.003
FKBP51 overexpression in the corticolimbic system stabilizes circadian rhythms
Abstract
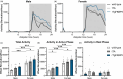
Circadian rhythm disruptions have been associated with a wide range of health issues and complications, including an increased risk of circadian rhythm sleep disorders (CRSDs). CRSDs are common among individuals who have been through a traumatic event, particularly in those who have post-traumatic stress disorder (PTSD). Allelic variations in the gene encoding for FK506-binding protein 51 (FKBP51) can increase the susceptibility for PTSD and other stress-related disorders following trauma. At least one of these variants increases the levels of FKBP51 following stress through a glucocorticoid receptor-mediated process. Here, we used a mouse model that overexpresses human FKBP51 throughout the forebrain, rTgFKBP5, to investigate if elevated FKBP51 contributes to circadian rhythm disruption. Surprisingly, our findings indicate a greater rhythm amplitude and decreased rhythm fragmentation in rTgFKBP5 mice, particularly females, compared to controls. Female rTgFKBP5 mice also showed higher corticosterone levels basally and following stress exposure. Overall, this study associates FKBP51 overexpression with beneficial circadian rhythm outcomes.
Keywords: Circadian clock; Circadian rhythm sleep disorders; FKBP5; FKBP51; Transgenic mouse.
Published by Elsevier Inc.
Conflict of interest statement
Declarations of interest The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: Laura Blair reports financial support was provided by the National Institutes of Health, the Alzheimer’s Association, and the US Department of Veterans Affairs. Laura Blair has patent #US20150327523 A1 issued to Laura Blair. Laura Blair is a Senior Editor at Cell Stress and Chaperones. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
FKBP51 functions in the regulation of circadian rhythm and Alzheimer's disease.Cell Stress Chaperones. 2025 Mar;30(2):81-83. doi: 10.1016/j.cstres.2025.02.002. Epub 2025 Feb 9. Cell Stress Chaperones. 2025. PMID: 39933601 Free PMC article. Review.
-
Fkbp5 gene deletion: Circadian rhythm profile and brain proteomics in aged mice.Aging Cell. 2024 Dec;23(12):e14314. doi: 10.1111/acel.14314. Epub 2024 Sep 3. Aging Cell. 2024. PMID: 39225086 Free PMC article.
-
Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency.PLoS One. 2014 Sep 5;9(9):e107241. doi: 10.1371/journal.pone.0107241. eCollection 2014. PLoS One. 2014. PMID: 25191701 Free PMC article.
-
Deficiency of FK506-binding protein (FKBP) 51 alters sleep architecture and recovery sleep responses to stress in mice.J Sleep Res. 2014 Apr;23(2):176-85. doi: 10.1111/jsr.12112. Epub 2013 Dec 5. J Sleep Res. 2014. PMID: 24354785
-
Genetically engineered mouse models of FK506-binding protein 5.J Cell Biochem. 2024 Dec;125(12):e30374. doi: 10.1002/jcb.30374. Epub 2023 Feb 13. J Cell Biochem. 2024. PMID: 36780339 Review.
Cited by
-
Glucocorticoid Insensitivity: Is It a Question of Time and Place?Biomedicines. 2025 Jun 10;13(6):1418. doi: 10.3390/biomedicines13061418. Biomedicines. 2025. PMID: 40564137 Free PMC article. Review.
-
FKBP51 functions in the regulation of circadian rhythm and Alzheimer's disease.Cell Stress Chaperones. 2025 Mar;30(2):81-83. doi: 10.1016/j.cstres.2025.02.002. Epub 2025 Feb 9. Cell Stress Chaperones. 2025. PMID: 39933601 Free PMC article. Review.
References
-
- Patke A., Young M.W., Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21:67–84. - PubMed
-
- Finger A.M., Dibner C., Kramer A. Coupled network of the circadian clocks: a driving force of rhythmic physiology. FEBS Lett. 2020;594:2734–2769. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

