Associations between hypothalamic-pituitary-adrenal (HPA) axis hormone levels, major depression features and antidepressant effects of ketamine
- PMID: 39674325
- PMCID: PMC12186659
- DOI: 10.1016/j.jad.2024.12.036
Associations between hypothalamic-pituitary-adrenal (HPA) axis hormone levels, major depression features and antidepressant effects of ketamine
Abstract
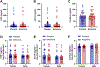
Background: Subanesthetic doses of (R,S)-ketamine (ketamine) have demonstrated rapid and robust antidepressant effects in individuals with depression. However, individual variability in response to ketamine exists, and current biomarkers of ketamine treatment response are not entirely understood. Preclinical evidence suggests a link between hypothalamic-pituitary-adrenal (HPA) axis activation, a determinant of the stress response system, and ketamine's efficacy in stressed mice exhibiting enhanced antidepressant responses. Here, we assessed the relationship between HPA axis, major depression features, and antidepressant response to ketamine in humans.
Methods: We investigated 42 participants following medication washout with treatment-resistant depression who participated in a randomized, placebo-controlled, crossover trial receiving intravenous ketamine. Plasma levels of corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH), and cortisol were measured at baseline. Ketamine's antidepressant effects were assessed using the Montgomery-Asberg Depression Rating Scale.
Results: We found that baseline HPA axis hormone levels did not significantly moderate the antidepressant effects of ketamine. However, a negative association was observed between ACTH and CRF levels and the overall duration of depressive episodes, suggesting potential biomarker implications. Also, a negative correlation between baseline depressive scores and age of onset was observed, suggesting that the severity of depression might be greater if it develops at a younger age, indicating more enduring stress on the brain and body.
Discussion: Although we did not find a moderation effect of the plasma HPA axis hormones on the antidepressant effects of ketamine, moderation effects of the brain HPA axis hormones cannot be precluded and warrants further investigation. Importantly, our results implicate HPA axis components as potential biomarkers for the duration of depressive episodes.
Keywords: Adrenocorticotropic hormone; Corticotropin releasing factor; Cortisol; Depression; Ketamine.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest Dr. Zarate is listed as a coinventor on a patent for the use of ketamine in major depression and suicidal ideation. Dr. Zarate is listed as coinventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Drs. Zarate, and Gould are listed as co-inventors on a patent application for the crystal forms and methods of synthesis of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine and the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. Dr. Zarate have assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Gould has assigned his patent rights to the University of Maryland. All other authors have no conflict of interest to disclose, financial or otherwise.
Figures

References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. - PubMed
-
- Zarate CAJ, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. - PubMed
-
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry. 2016;173:816–826. - PubMed
-
- Frazer A, Benmansour S. Delayed pharmacological effects of antidepressants. Mol Psychiatry. 2002;7 Suppl 1:S23–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

