Targeting the neonatal Fc receptor (FcRn) is not beneficial in an animal model of chronic neuritis
- PMID: 39674966
- PMCID: PMC11646953
- DOI: 10.1007/s12026-024-09565-7
Targeting the neonatal Fc receptor (FcRn) is not beneficial in an animal model of chronic neuritis
Abstract
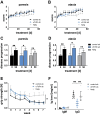
The inhibition of the neonatal Fc receptor (FcRn) is a promising therapeutic pathway in certain autoimmune disorders to reduce the amount of circulating pathogenic IgG autoantibodies by interfering with their recycling system. FcRn antibodies are currently being tested in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). This study aimed to investigate the therapeutic potential of an antibody targeting FcRn in the intracellular adhesion molecule 1 (ICAM1)-deficient NOD mouse-a model representative for many aspects of human CIDP. After the onset of clinical signs of neuropathy, ICAM1-deficient NOD mice were assigned to treatment twice per week with anti-FcRn antibody, isotype control antibody (negative control) or intraperitoneal (administered) immunoglobulin (positive control). Disease severity was monitored using disease-specific assessments for ataxia and paresis such as grip strength measurements. Serum immunoglobulin levels and peripheral nerve immune cell infiltration were quantified. Treatment with anti-FcRn antibody did not ameliorate disease progression, as determined by clinical scores and grip strength analysis. Disease progression was reduced in the positive control animals receiving immunoglobulin. Consistent with the clinical results, the composition of infiltrating immune cells was not altered in the peripheral nerve of anti-FcRn antibody-treated mice compared to controls. However, in anti-FcRn antibody-treated mice, significantly lower IgG levels were detectable compared to controls. These findings suggest that targeting the FcRn recycling system does not influence disease progression in the NOD-ICAM1-deficient mouse model of CIDP. Further studies will elucidate whether the reduction of IgG levels was insufficient to deplete pathogenic autoantibodies or whether the major inflammatory driver in the NOD-ICAM1-deficient mouse animal model is mediated by factors other than pathological immunoglobulins.
Keywords: Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP); ICAM-deficient NOD mouse; Immune-mediated neuropathy; Intravenous immunoglobulins (IVIg); Neonatal Fc receptor (FcRn).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Not applicable. Consent for publication: The authors all signed written consent for data publication. Conflict of interest: The authors declare no competing interests.
Figures

References
-
- Kieseier BC, Mathey EK, Sommer C, Hartung HP. Immune-mediated neuropathies. Nat Rev Dis Prim. 2018;4(1):3110.1038/s41572-018-0027-2 - PubMed
-
- Hughes RAC, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–44. 10.1016/S1474-4422(07)70329-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

