Prospective observational study of FKRP-related limb-girdle muscular dystrophy R9: A GRASP consortium study
- PMID: 39675022
- PMCID: PMC11822816
- DOI: 10.1002/acn3.52276
Prospective observational study of FKRP-related limb-girdle muscular dystrophy R9: A GRASP consortium study
Abstract
Objective: Limb-girdle muscular dystrophy R9 (LGMDR9, formerly known as LGMD2I), caused by variants in the fukutin-related protein (FKRP) gene leads to progressive muscle weakness of the shoulder and pelvic limb-girdles and loss of motor function over time. Clinical management and future trial design are improved by determining which standardized clinical outcome assessments (COA) of function are most appropriate to capture disease presentation and progression, informing endpoint selection and enrollment criteria. The purpose of our study was to evaluate the cross-sectional validity and reliability of clinical outcome assessments in patients with FKRP-related LGMDR9 participating in the Genetic Resolution and Assessments Solving Phenotypes in LGMD (GRASP) natural history study.
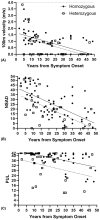
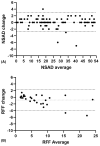
Methods: Enrolled patients completed a battery of COA on two consecutive days, including the North Star Assessment for limb girdle-type dystrophies (NSAD), the 100-m timed test (100 m), and the Performance of Upper Limb 2.0 (PUL).
Results: A total of 101 patients with FKRP-related LGMDR9 completed COA evaluations. All functional COA were highly and significantly correlated even across constructs, except for the 9-hole peg test. Similarly, all tests demonstrated excellent test-retest reliability across 2-day visits. The NSAD and PUL demonstrate robust psychometrics with good targeting, ordered response thresholds, fit and stability, and limited dependency of items across the scales.
Conclusions: This study has determined the suitability of several functional COA, cross-sectionally, in LGMDR9 to inform future trial design and clinical care.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Dr. Alfano received consultant fees from Asklepios Biopharmaceutical and ML Bio Solutions via ATOM International and received funding via institution and royalties from Sarepta Therapeutics. Dr. James receives consulting fees from ATOM International (covers consultancy services provided to Genethon, PTC Therapeutics, Sarepta Therapeutics) and consultant fees related to advisory boards via Newcastle University for Sarepta Therapeutics. Dr. Vissing received consultant fees for serving on advisory boards for Sarepta Therapeutics, ML Bio Solutions, and Atamyo. Dr. Mozaffar received consultant fees for serving in an advisory capacity for Ask Bio and research funding from ML Bio Solutions. Dr. Lowes receives funding via institution and royalties from Sarepta Therapeutics. Dr. Weihl received consultant fees for advising ML Bio Solutions and Sarepta Therapeutics. Dr. Kang received research support from ML Bio Solutions and Sarepta Therapeutics. Dr. Johnson received research funds from AskBio, ML Bio Solutions, and Sarepta Therapeutics. He has received consulting fees AskBio. Dr. Johnson has stock options in Repeat RNA Therapeutics, Angle Therapeutics, and Myogene Therapies. KGP, KR, RA, AJ, AB, KML, SRHM, KDM, MAI, NFR, LP, TS, MW, SH, SS, MC, JMS, NS, MH, DGL, DJL, PBK, SH, UD have nothing to report.
Figures

References
-
- Mercuri E, Brockington M, Straub V, et al. Phenotypic spectrum associated with mutations in the fukutin‐related protein gene. Ann Neurol. 2003;53(4):537‐542. - PubMed
-
- Poppe M, Cree L, Bourke J, et al. The phenotype of limb‐girdle muscular dystrophy type 2I. Neurology. 2003;60(8):1246‐1251. - PubMed
-
- Richard I, Laurent JP, Cirak S, et al. 216th ENMC international workshop: clinical readiness in FKRP related myopathies January 15–17, 2016 Naarden. The Netherlands Neuromuscul Disord. 2016;26(10):717‐724. - PubMed
-
- Jensen SM, Muller KI, Mellgren SI, et al. Epidemiology and natural history in 101 subjects with FKRP‐related limb‐girdle muscular dystrophy R9. The Norwegian LGMDR9 cohort study (2020). Neuromuscul Disord. 2023;33(2):119‐132. - PubMed

