Bleed treatment with eptacog beta (rFVIIa) results in a low incidence of rebleeding in adult and adolescent patients with haemophilia A or B with inhibitors
- PMID: 39676340
- PMCID: PMC11780187
- DOI: 10.1111/hae.15109
Bleed treatment with eptacog beta (rFVIIa) results in a low incidence of rebleeding in adult and adolescent patients with haemophilia A or B with inhibitors
Abstract
Introduction: Eptacog beta is a novel human recombinant FVIIa approved for use in the United States, European Union, United Kingdom and Mexico for the treatment and control of bleeding in patients with haemophilia A or B with inhibitors (≥12 years). It is also indicated for perioperative care in the same patient population in Europe and the United Kingdom.
Aim: To assess the incidence of rebleeding and review treatment outcomes in subjects with haemophilia with inhibitors enrolled in the phase 3 PERSEPT 1 clinical trial.
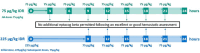
Methods: To treat mild/moderate bleeding episodes (BEs), subjects administered an initial 75 or 225µg/kg dose of eptacog beta, followed (if necessary) by additional 75µg/kg doses at predefined intervals until bleed control. This analysis used subject-reported rebleeding to determine a rebleeding incidence for the first 24 h. Rebleeding through later timepoints was an exploratory, intention-to-treat analysis of bleed treatment data.
Results: Four hundred and sixty-five BEs were analysed. Through 24 h, the proportion of rebleeds was 0% (initial 75µg/kg dose) and 0.5% (initial 225µg/kg dose). Through 48 h, the proportion of rebleeds was 3.2% (75µg/kg initial dose) and 5.6% (225µg/kg initial dose); the difference between initial dose strategies was not statistically significant. The majority of rebleeds were controlled with a single dose of eptacog beta and no subject who treated a rebleed required hospitalization.
Conclusion: Subjects with haemophilia with inhibitors who used eptacog beta to treat mild/moderate BEs experienced a low incidence of rebleeding. Rebleeds that did occur were effectively controlled with eptacog beta (median, one dose) without the need for hospitalization.
Keywords: efficacy; eptacog beta; haemophilia; inhibitors; rebleeding incidence; recombinant FVIIa.
© 2024 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
Amy Dunn has received consulting fees from Genentech, Kedrion, CSL Behring, BioMarin, uniQure, Sanofi, Novo Nordisk and HEMA Biologics; has received research funding from Takeda, BioMarin, Freeline, Novo Nordisk, Sanofi, Pfizer, Genentech and ATHN; is on the board of the World Federation of Hemophilia USA; is Chair of MASAC of the National Bleeding Disorders Foundation; and is on the Board of Cascade. Yesim Dargaud has received research funding from CSL Behring, Novo Nordisk and LFB; has served as a member on the Board of Directors or advisory committees for CSL Behring, Sobi, LFB, BioMarin and Pfizer; and has served as a member of an expert board for CSL Behring, Sobi, LFB, BioMarin, and Pfizer. Yasmina Abajas has acted as a paid consultant to Sanofi. Manuel Carcao has received research funding from Bioverativ/Sanofi, Novartis, Novo Nordisk, Pfizer, Roche and Shire/Takeda; and has received honoraria from Bayer, Bioverativ/Sanofi, LFB, Novo Nordisk, Pfizer, Roche and Shire/Takeda. Giancarlo Castaman has received honoraria as a speaker/participant on advisory boards for Bayer, Takeda, CSL Behring, Novo Nordisk, Sobi, Roche, Pfizer, LFB, BIOVIIIx and BioMarin. Adam Giermasz has served as a consultant or advisory board member for Sanofi, HEMA Biologics, Vega Therapeutics, Alexion and Genzyme; has been a member of DSMC committee for Adrenas, and has acted as a speaker for Sanofi, Alexion and Genzyme. Cédric Hermans has received research funding from Bayer, BioMarin, CSL Behring, Novo Nordisk, Pfizer, Shire/Takeda and Sobi; and has received honoraria and speakers’ bureau fees from LFB, Bayer, CAF‐DCF, CSL Behring, Hoffmann‐La Roche, LFB, Novo Nordisk, Octapharma, Pfizer, Shire/Takeda, Sobi and uniQure. Victor Jiménez‐Yuste has received consulting fees from Grifols, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Takeda, Bayer, CSL Behring, Pfizer, BioMarin, Sanofi and Sobi; has received honoraria from Grifols, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Takeda, Bayer, CSL Behring, Pfizer, Sanofi and Sobi; and has received research funding from Grifols, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Takeda, Bayer, CSL Behring, Pfizer, Sanofi and Sobi. Magdalena Lewandowska has no competing interests to declare. Johnny Mahlangu has received research grants from BioMarin, Catalyst Biosciences, CSL, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Spark and uniQure; has served as a consultant or member of the scientific board for BioMarin, CSL Behring, Catalyst Biosciences, Novo Nordisk, Roche, Sanofi, Spark and Takeda; and has received speaker bureau fees from ISTH, Novo Nordisk, Pfizer, Roche, Sanofi, Takeda and WFH. Shannon Meeks has received payment as a member of medical and scientific advisory boards for BioMarin, CSL Behring, Genentech and Sanofi; has acted as a paid consultant for Spark, Pfizer and Teraimmune; and has received research funding from Genentech and Octapharma. Wolfgang Miesbach has acted a consultant for Bayer, BioMarin, Biotest, CSL Behring, Chugai, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Regeneron, Roche, Sanofi, Sigilon, Sobi, Takeda/Shire, uniQure. Michael Recht's employers have received research funding from Bayer, BioMarin, CSL Behring, Genentech, Grifols, HEMA Biologics, LFB, NovoNordisk, Octapharma, Pfizer, Sanofi, Spark, Takeda and uniQure; has served as a paid advisor to CSL Behring, Genentech, HEMA Biologics, Kedrion, NovoNordisk, Pfizer, Sanofi, Takeda and uniQure; and is on the Board of Directors of Partners in Bleeding Disorders. Vanessa Salinas has received consulting fees from Bayer, Genentech, and Sanofi; and has served on a speakers’ bureau for Bayer, Sanofi and Genentech. Tammuella Chrisentery‐Singleton has received consulting fees from Sanofi, GBT, Octapharma, Genentech, BioMarin, HEMA Biologics, Novo Nordisk, Takeda, BPL, CSL Behring, Pfizer, Bayer, Grifols and Kedrion; has received honoraria from Sanofi, GBT, Octapharma, Genentech, BioMarin, HEMA Biologics, Novo Nordisk, Takeda, BPL, CSL Behring, Pfizer, Bayer, Grifols and Kedrion; has participated in a speakers bureau for Sanofi, GBT, Octapharma, Genentech, BioMarin, HEMA Biologics, Novo Nordisk, Takeda, BPL, CSL Behring and Pfizer; and has received research funding from Pfizer. Daniel Bonzo is an employee of LFB‐USA, Inc. Ian S. Mitchell is an employee of HEMA Biologics and US WorldMeds; he was formerly a consultant with HEMA Biologics. Thomas A. Wilkinson is an analyst/medical writer for GLOVAL LLC that has received consulting fees from HEMA Biologics and LFB. Guy Young has received consulting fees from BioMarin, Centessa, CSL Behring, Genentech/Roche, HEMA Biologics/LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Genzyme, Spark and Takeda; and has received funds for research support from Genentech/Roche, Sanofi and Takeda.
Figures


References
-
- Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369:2313‐2323. - PubMed
-
- von Drygalski A, Chowdary P, Kulkarni R, et al. Efanesoctocog Alfa Prophylaxis for Patients with Severe Hemophilia A. N Engl J Med. 2023;388:310‐318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

