Diagnosis, treatment, management and monitoring of patients with tyrosinaemia type 1: Consensus group recommendations from the German-speaking countries
- PMID: 39676394
- PMCID: PMC11647197
- DOI: 10.1002/jimd.12824
Diagnosis, treatment, management and monitoring of patients with tyrosinaemia type 1: Consensus group recommendations from the German-speaking countries
Abstract
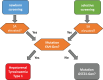
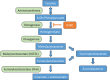
Hepatorenal tyrosinaemia (HT1) is an autosomal recessive disorder of tyrosine degradation resulting in hepatic and renal dysfunction, neurological sequelae may occur in some patients. The use of nitisinone (NTBC) has revolutionised treatment and outcome of this disorder. NTBC has to be combined with a low protein diet. While NTBC modulates the disease course in HT1 patients, several issues are open. Optimal dosage, doses per day, therapeutic range of NTBC concentration, mode of protein restriction and biomarkers are not well defined. HCC and neurocognitive deficits are long-term sequelae. Early diagnosis and treatment are essential to minimise the risk for these complications. Clinical guidance for management of HT1-patients is required. Randomised clinical studies are difficult in the presence of therapeutic options. We discussed these issues in a consensus group of 10 paediatricians, 1 adult hepatologist, 1 geneticist, 2 dieticians, 2 newborn screening specialists with experience in HT1, 1 psychologist and 2 representatives of a patient group from the German-speaking countries (DACH). Recommendations were based on scientific literature and expert opinion, also taking into account recent experience with newborn screening. There was strong consensus that newborn screening using succinylacetone (SA) and early treatment are essential for a good outcome. The dose of NTBC should be as low as possible without losing metabolic control. This has to be accompanied by a low protein diet, in some patients a simplified diet without calculation of protein intake. Specific education and psychosocial support are recommended. Indications for liver transplantation were defined. Monitoring shall include clinical findings, levels of SA, tyrosine, phenylalanine and NTBC in (dried) blood.
Keywords: hepatorenal tyrosinaemia; newborn screening; nitisinone; succinylacetone; tyrosine.
© 2024 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Anibh M. Das, Diana Ballhausen, Dorothea Haas, Johannes Häberle, Tobias Hagedorn, Cecilia Janson‐Mutsaerts, Nils Janzen, Johannes Sander, Peter Freisinger, Daniela Karall, Uta Meyer, Eberhard Mönch, Susanne Morlot, Stefanie Rosenbaum‐Fabian, Sabine Scholl‐Bürgi, Stephan vom Dahl, Natalie Weinhold, Jiri Zeman, and Karin Lange declare that they have no conflict of interest.
Figures
References
-
- Poudrier J, St‐Louis M, Lettre F, et al. Frequency of the IVS12 + 5G‐‐>A splice mutation of the fumarylacetoacetate hydrolase gene in carriers of hereditary tyrosinaemia in the French Canadian population of Saguenay‐Lac‐St‐Jean. Prenat Diagn. 1996;16(1):59‐64. doi:10.1002/(SICI)1097-0223(199601)16:1<59::AID-PD810>3.0.CO;2-D - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

