Evaluation of Alternative Lithium Salts for High-Voltage Lithium Ion Batteries: Higher Relevance of Plated Li Morphology Than the Amount of Electrode Crosstalk
- PMID: 39676517
- PMCID: PMC12067173
- DOI: 10.1002/smll.202410762
Evaluation of Alternative Lithium Salts for High-Voltage Lithium Ion Batteries: Higher Relevance of Plated Li Morphology Than the Amount of Electrode Crosstalk
Abstract
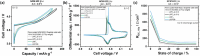
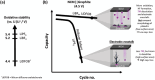
Increasing the upper cut-off voltage (UCV) enhances the specific energy of Li-ion batteries (LIBs), but is accompanied by higher capacity fade as a result of electrode cross-talk, i.e., transition metals (TM) dissolution from cathode and deposition on anode, finally triggering high surface area lithium (HSAL) formation due to locally enhanced resistance. Here, LiPF6, LiBF4, lithium difluoro(oxalate)borate (LiDFOB), lithium bis(oxalate)borate (LiBOB), lithium bis(fluorosulfonyl)imide (LiFSI), and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in carbonate-based solvents are investigated in LiNi0.6Co0.2Mn0.2O2 (NCM 622) || graphite pouch cells with 4.5 V UCV. Despite the lower oxidative stabilities of LiBF4 and LiDFOB, thus enhanced HF formation, TM dissolution, and consequently electrode cross-talk, higher capacity retention is observed compared to the case of LiPF6 electrolyte. Counterintuitively, it is not the TM deposit amount but rather the Li plating morphology that governs capacity fade, as these salts cause more uniform and compact lithium plating, i.e., lower surface area. In contrast, the dendritic HSAL with LiPF6 has a higher surface area, and more parasitic reactions, thus active Li ("Li inventory") losses and capacity fade. Although NCM initiates the failure cascade, the capacity losses and cycle life of high-voltage LIBs are predominantly determined by the anode, in particular the Li plating morphology and the corresponding surface area.
Keywords: cross‐talk; full‐cell; high surface area lithium (HSAL) plating; high‐voltage operation; lithium battery; lithium conducting salts; lithium ion battery; rollover failure; transition metal dissolution.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Schmuch R., Wagner R., Hörpel G., Placke T., Winter M., Nat. Energy 2018, 3, 267.
-
- Noh H.‐J., Youn S., Yoon C. S., Sun Y.‐K., J. Power Sources 2013, 233, 121.
-
- Kasnatscheew J., Wagner R., Winter M., Cekic‐Laskovic I., Top. Curr. Chem. 2018, 376, 16. - PubMed
-
- Kasnatscheew J., Evertz M., Streipert B., Wagner R., Nowak S., Cekic Laskovic I., Winter M., J. Power Sources 2017, 359, 458.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

