Clinical Trial: Safety and Efficacy of a Novel Oesophageal Delivery System for Topical Corticosteroids Versus Placebo in the Treatment of Eosinophilic Oesophagitis
- PMID: 39676687
- PMCID: PMC11707640
- DOI: 10.1111/apt.18443
Clinical Trial: Safety and Efficacy of a Novel Oesophageal Delivery System for Topical Corticosteroids Versus Placebo in the Treatment of Eosinophilic Oesophagitis
Abstract
Background: EsoCap is a thin mucoadhesive film designed to target the oesophageal mucosa. The device loaded with mometasone furoate (ESO-101) is under investigation for the treatment of eosinophilic oesophagitis (EoE).
Aims: To evaluate the efficacy, safety and tolerability of ESO-101 in patients with active EoE.
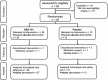
Methods: We conducted a randomised, placebo-controlled, phase 2, proof-of-concept trial at 14 European sites in adults with EoE. Participants received placebo, uncoated EsoCap (n = 15), or EsoCap loaded with 800 μg of mometasone furoate (n = 28) once daily during 28 days. The primary outcome was the absolute change in the peak eosinophil count; secondary outcomes were histologic, clinical and endoscopic measures.
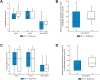
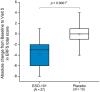
Results: Treatment with ESO-101 resulted in reduction (mean ± SD) of 49.1 ± 88.4 eosinophils/high-power field from baseline, compared with 6.6 ± 65.1 with placebo (p = 0.03). With ESO-101, 48% and 44% of patients achieved < 15 and < 6 eosinophils/high-power field, respectively; these were 0% with placebo. EoE Endoscopic Reference Score reduced significantly in patients treated with ESO-101. In contrast, dysphagia and odynophagia severity decreased similarly in both groups. There were no serious treatment-emergent adverse events. Mean serum cortisol did not change significantly throughout the trial. Notably, no oropharyngeal or oesophageal candidiasis was documented. The device was well tolerated.
Conclusions: ESO-101 was superior to placebo in reducing oesophageal eosinophilia. The device was safe and well tolerated in adults with EoE, supporting the continued development of ESO-101 for the treatment of EoE (Trials.gov No.: NCT04849390; Eu-CT No.: 2020-000082-16).
Keywords: corticosteroid; drug delivery; eosinophilic oesophagitis; histopathology; topical therapy.
© 2024 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
A.J.L. receives research funding from Adare/Ellodi, AstraZeneca, Dr. Falk Pharma, EsoCap, and Regeneron and serves as a consultant for Alfasigma, Arena/Pfizer, EsoCap, Dr. Falk Pharma, EsoCap, and Sanofi. O.N.C. received funding for scientific activities from Abbvie, Falk, Takeda, Adacyte, Shire, Faes‐Farma, AstraZeneca, Pfizer, Janssen. A.S. has consultant contracts Astra‐Seneca, BMS‐Receptos, Calypso, EsoCap, Falk Pharma, GSK, Pfizer, Sanofi‐Regeneron. L.B.: Advisory for Abbvie, Amgen, BMS, Falk, Janssen, Pfizer, Lilly, Takeda, Sanofi, Esocap; Speaker for Takeda, Sanofi, Abbvie, Lilly, Dr. Falk Pharma, BMS, and Pfizer. A.J.B. received research funding from BMS, Sanofi/Regeneron, SST, MMS, and Dr. Falk Pharma and received speaker and/or consulting fees from Uniquity, Laborie, Medtronic, BMS, Dr. Falk Pharma, Calypso Biotech, Eupraxia, Aqilion, Alimentiv, Sanofi/Regeneron, Uniquity, Reckitt, AlfaSigma and AstraZeneca. D.G. receives funding for scientific activities from Tillots Pharma, Falk, Takeda, Sanofi Biotechnology, Dr. Falk Pharma, Laboratorio Salvat. S.W. received speaker and/or consulting fees from Janssen, Sanofi, AstraZeneca, MSD, Tillotts, Gilead, Abbvie, Johnson&Johnson, Takeda, Dr. Falk Pharma, and BMS. U.v.A. received speaker and/ or consulting fess from BMS, Dr. Falk Pharma, Sanofi/ Regerenon, Alfasigma, Pfizer, Lilly, Abbive, Takeda, Esocap, Johnson&Johnson, Astra Zeneca. J.B. served as a speaker, as a consultant or received research or education funding from Abbvie, Takeda, Janssen, Kern Pharma and Ferring. M.V. received honoraria as speaker from Dr. Falk Pharma and Esocap. E.S.D. receives research funding from Adare/Ellodi, Allakos, Arena/Pfizer, AstraZeneca, Celldex, Eupraxia, Ferring, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Revolo, Sanofi, and Shire/Takeda. He is a consultant for Abbvie, Adare/Ellodi, Akesobio, Alfasigma, ALK, Allakos, Amgen, Apollo, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biocryst, Bryn, Calypso, Celgene/Receptos/BMS, Celldex, EsoCap, Eupraxia, Dr. Falk Pharma, Ferring, GI Reviewers, GSK, Holoclara, Invea, Knightpoint, LucidDx, Morphic, Nexstone Immunology/Uniquity, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Sanofi, Shire/Takeda, Target RWE, Upstream Bio. Educational grant provided by Allakos, Aqilion, Holoclara, Invea. The remaining authors have nothing to disclose.
Figures



References
-
- Laserna‐Mendieta E. J., Casabona S., Guagnozzi D., et al., “Efficacy of Proton Pump Inhibitor Therapy for Eosinophilic Oesophagitis in 630 Patients: Results From the EoE Connect Registry,” Alimentary Pharmacology & Therapeutics 52 (2020): 798–807. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
