Reference Ranges and Determinants of Thyroid Function and TSH Receptor Antibodies During Early Pregnancy in Nepal
- PMID: 39676726
- PMCID: PMC11788947
- DOI: 10.1111/cen.15175
Reference Ranges and Determinants of Thyroid Function and TSH Receptor Antibodies During Early Pregnancy in Nepal
Abstract
Objective: Different definitions of thyroid dysfunction during pregnancy may lead to under or overtreatment. The aims of this study were to (1) define population-based pregnancy-specific reference ranges for thyroid dysfunction during early pregnancy in Nepal and assess the impact of antibody positivity, (2) quantify the diagnostic impact of population-based reference ranges compared with current practice and (3) assess the determinants of thyroid function and antibody positivity.
Methods: A total of 800 healthy pregnant women aged 20-40 years in the Bhaktapur municipality were included. Population-based reference ranges for thyroid hormones levels were defined as 2.5th and 97.5th percentiles using competitive immunoluminometric assay design. Thyroid disease cases and those with recommended treatment indications were calculated using current non-pregnancy new reference ranges. Multivariate regression analyses were performed to identify the determinants of thyroid hormones and antibody levels.
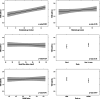
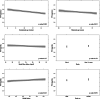
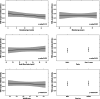
Results: Median gestational age was 11 weeks. The reference interval was 0.05-3.69 µLU/mL for thyroid stimulating hormone (TSH) and 8.89-15.28 pg/mL for free tetraiodothyronine (fT4) after excluding thyroid peroxidase antibody-positive women. Compared with the current non-pregnancy reference ranges, the new calculations increased the number of women who required treatment from 5 to 12 (0.9% increase). We identified 19 women (2.4%) who were positive for TSH receptor antibody (TRAb). We could not identify the determinants of TRAb positivity, and TRAb positivity was not associated with TSH or fT4 levels.
Conclusions: We found meaningful changes using population-based pregnancy-specific TSH and fT4 reference intervals and encourage further studies in other low- and middle-income settings. Our findings suggest that population screening for TRAb is not clinically meaningful.
Trial registration: Clinical Trial Registration: U1111-1183-4093.
Keywords: Nepal; TPOAb; TRAb; TgAb; pregnancy; reference range; thyroid autoimmunity; thyroid function.
© 2024 The Author(s). Clinical Endocrinology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

