Characterization of Spirulina-derived extracellular vesicles and their potential as a vaccine adjuvant
- PMID: 39676887
- PMCID: PMC11635480
- DOI: 10.1002/jex2.70025
Characterization of Spirulina-derived extracellular vesicles and their potential as a vaccine adjuvant
Abstract
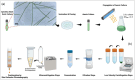
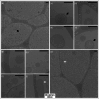
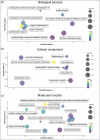
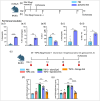
Spirulina is an edible cyanobacterium that increasingly gaining recognition for it untapped potential in the biomanufacturing of pharmaceuticals. Despite the rapidly accumulating information on extracellular vesicles (EVs) from most other bacteria, nothing is known about Spirulina extracellular vesicles (SPEVs). This study reports the successful isolation, characterization and visualization of SPEVs for the first time and it further investigates the potential therapeutic benefits of SPEVs using a mouse model. SPEVs were isolated using ultracentrifugation and size-exclusion-chromatography. Cryo-Transmission Electron Microscopy revealed pleomorphic outer-membrane-vesicles and outer-inner-membrane-vesicles displaying diverse shapes, sizes and corona densities. To assess short- and long-term immune responses, mice were injected intraperitoneally with SPEVs, which demonstrated a significant increase in neutrophils and M1 macrophages at the injection site, indicating a pro-inflammatory effect induced by SPEVs without clinical signs of toxicity or hypersensitivity. Furthermore, SPEVs demonstrated potent adjuvanticity by enhancing antigen-specific IgG responses in mice by over 100-fold compared to an unadjuvanted model vaccine antigen. Mass-spectrometry identified 54 proteins within SPEVs, including three protein superfamily members linked to the observed pro-inflammatory effects. Our findings highlight the potential of SPEVs as a new class of vaccine adjuvant and warrant additional studies to further characterize the nature of the immune response.
Keywords: adjuvant; extracellular vesicle; immune response; spirulina; vaccine.
© 2024 The Author(s). Journal of Extracellular Biology published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Adamo, G. , Fierli, D. , Romancino, D. P. , Picciotto, S. , Barone, M. E. , Aranyos, A. , Božič, D. , Morsbach, S. , Raccosta, S. , Stanly, C. , Paganini, C. , Gai, M. , Cusimano, A. , Martorana, V. , Noto, R. , Carrotta, R. , Librizzi, F. , Randazzo, L. , Parkes, R. , … Bongiovanni, A. (2021). Nanoalgosomes: Introducing extracellular vesicles produced by microalgae. Journal of Extracellular Vesicles, 10(6), e12081. 10.1002/jev2.12081 - DOI - PMC - PubMed
-
- Ausiello, C. M. , Cerquetti, M. , Fedele, G. , Spensieri, F. , Palazzo, R. , Nasso, M. , Frezza, S. , & Mastrantonio, P. (2006). Surface layer proteins from Clostridium difficile induce inflammatory and regulatory cytokines in human monocytes and dendritic cells. Microbes and Infection, 8(11), 2640–2646. 10.1016/j.micinf.2006.07.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
