Efficacy and Safety of Farletuzumab in Ovarian Cancer: A Systematic Review and Single-Arm Meta-Analysis
- PMID: 39677200
- PMCID: PMC11638381
- DOI: 10.7759/cureus.73503
Efficacy and Safety of Farletuzumab in Ovarian Cancer: A Systematic Review and Single-Arm Meta-Analysis
Abstract
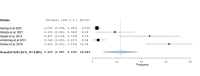
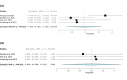
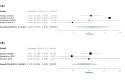
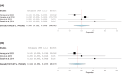
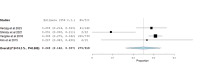
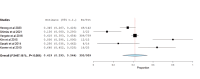
Folate receptor alpha (FRα) has emerged as a promising target in the treatment of ovarian cancer, with farletuzumab, a humanized monoclonal antibody targeting FRα, showing potential in clinical settings. This systematic review and single-arm meta-analysis aimed to evaluate the efficacy and safety of farletuzumab in patients with solid tumors, particularly ovarian cancer. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we conducted a thorough search across PubMed, the Web of Science, Scopus, and the Cochrane Central Register of Controlled Trials (CENTRAL) for clinical trials assessing farletuzumab in solid tumors. Data were extracted on study characteristics, patient demographics, treatment regimens, and efficacy outcomes including progression-free survival (PFS), overall survival (OS), response rates, and adverse events (AEs). The pooled analyses were performed using the Open Meta-Analyst software. In total, seven prospective studies were included, covering various farletuzumab regimens in ovarian cancer and other solid tumors. The pooled PFS was 10.5 months (95% CI: 8, 15.7) across three studies involving 925 patients, while the pooled OS was 36.7 months (95% CI: 26.6, 35) in two studies with 881 patients. Treatment response rates indicated a partial response in 55.25% of patients and stable disease in 28.68% of cases. Gastrointestinal and hematological AEs were frequently reported, with nausea (52.14%), neutropenia (50.65%), and anemia (39.76%) being the most common. Farletuzumab appears to offer a promising efficacy profile, particularly in ovarian cancer, with notable improvements in disease progression and survival. However, the treatment is associated with a high incidence of gastrointestinal and hematological AEs, raising the need for careful patient selection. Further studies are required to refine the therapeutic regimen and ensure an optimal balance between efficacy and safety.
Keywords: farletuzumab; frα; humanized monoclonal antibody; oncology; ovarian cancer.
Copyright © 2024, Fayoud et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures












References
-
- A human membrane-associated folate binding protein is anchored by a glycosyl-phosphatidylinositol tail. Luhrs CA, Slomiany BL. https://pubmed.ncbi.nlm.nih.gov/2557328/ J Biol Chem. 1989;264:21446–21449. - PubMed
-
- Recent advances in the understanding of the mechanism of membrane transport of folates and antifolates. Sierra EE, Goldman ID. https://pubmed.ncbi.nlm.nih.gov/10598550/ Semin Oncol. 1999;26:11–23. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
