Goldfish phenomics reveals commonalities and a lack of universality in the domestication process for ornamentation
- PMID: 39677575
- PMCID: PMC11637523
- DOI: 10.1093/evlett/qrae032
Goldfish phenomics reveals commonalities and a lack of universality in the domestication process for ornamentation
Abstract
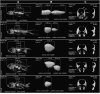
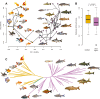
Domestication process effects are manifold, affecting genotype and phenotype, and assumed to be universal in animals by part of the scientific community. While mammals and birds have been thoroughly investigated, from taming to intensive selective breeding, fish domestication remains comparatively unstudied. The most widely bred and traded ornamental fish species worldwide, the goldfish, underwent the effect of long-term artificial selection on differing skeletal and soft tissue modules through ornamental domestication. Here, we provide a global morphological analysis in this emblematic ornamental domesticated fish. We demonstrate that goldfish exhibit unique morphological innovations in whole-body, cranial, and sensory (Weberian ossicles and brain) anatomy compared to their evolutionary clade, highlighting a remarkable morphological disparity within a single species comparable to that of a macroevolutionary radiation. In goldfish, as in the case of dogs and pigeons in their respective evolutionary contexts, the most ornamented varieties are extremes in the occupied morphological space, emphasizing the power of artificial selection for nonadaptive traits. Using 21st century tools on a dataset comprising the 16 main goldfish breeds, 23 wild close relatives, and 39 cypriniform species, we show that Charles Darwin's expressed wonder at the goldfish is justified. There is a commonality of overall pattern in the morphological differentiation of domesticated forms selected for ornamental purposes, but the singularity of goldfish occupation and extension within (phylo)morphospaces, speaks against a universality in the domestication process.
Keywords: artificial selection; fish domestication; phenomics.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Society for the Study of Evolution (SSE) and European Society for Evolutionary Biology (ESEN).
Figures




References
-
- Abe, G., Lee, S. H., Chang, M., Liu, S. C., Tsai, H. Y., & Ota, K. G. (2014). The origin of the bifurcated axial skeletal system in the twin-tail goldfish. Nature Communications, 5(1), 3360. https://doi.org/10.1038/ncomms4360 - DOI - PMC - PubMed
-
- Amundsen, T., & Forsgren, E. (2001). Male mate choice selects for female coloration in a fish. Proceedings of the National Academy of Sciences of the United States of America, 98(23), 13155–13160. https://doi.org/10.1073/pnas.211439298 - DOI - PMC - PubMed
-
- Anderson, M. J. (2001). A new method for non‐parametric multivariate analysis of variance. Austral Ecology, 26(1), 32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x - DOI
-
- Balcarcel, A. M., Geiger, M., Clauss, M., & Sánchez‐Villagra, M. R. (2022). The mammalian brain under domestication: Discovering patterns after a century of old and new analyses. Journal of Experimental Zoology. Part B. Molecular and Developmental Evolution, 338(8), 460–483. https://doi.org/10.1002/jez.b.23105 - DOI - PMC - PubMed
-
- Barton, R. A. (2012). Embodied cognitive evolution and the cerebellum. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences, 367(1599), 2097–2107. https://doi.org/10.1098/rstb.2012.0112 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

