This is a preprint.
Trehalose supports the growth of Aedes aegypti cells and modifies gene expression and dengue virus replication
- PMID: 39677712
- PMCID: PMC11643125
- DOI: 10.1101/2024.12.03.626538
Trehalose supports the growth of Aedes aegypti cells and modifies gene expression and dengue virus replication
Update in
-
Trehalose supports the growth of Aedes aegypti cells and modifies gene expression and dengue virus type 2 replication.PLoS Pathog. 2025 May 6;21(5):e1012795. doi: 10.1371/journal.ppat.1012795. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40327709 Free PMC article.
Abstract
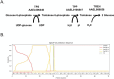
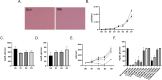
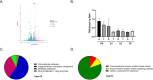
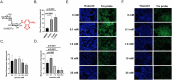
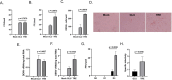
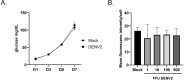
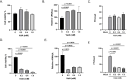
Trehalose is a non-reducing disaccharide that is the major sugar found in insect hemolymph fluid. Trehalose provides energy, and promotes growth, metamorphosis, stress recovery, chitin synthesis, and insect flight. Trehalase is the only enzyme responsible for the hydrolysis of trehalose, which makes it an attractive molecular target. Here we show that Aedes aegypti (Aag2) cells express trehalase and that they can grow on trehalose-containing cell culture media. Trehalase activity was confirmed by treating Aag2 cells with trehalase inhibitors, which inhibited conversion of trehalose to glucose and reduced cell proliferation. Cell entry of a fluorescent trehalose probe was dependent on trehalose concentration, suggesting that trehalose moves across the cell membrane via passive transport. Culturing Aag2 cells with trehalose-containing cell culture media led to significant changes in gene expression, intracellular lipids, and dengue virus replication and specific infectivity, and increased their susceptibility to trehalase inhibitors. These data describe an in vitro model that can be used to rapidly screen novel trehalase inhibitors and probes and underscores the importance of trehalose metabolism in Ae. aegypti physiology and transmission of a mosquito-borne virus.
Figures


References
-
- Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158(7):1445–59. - PubMed
-
- Dighe SN, Ekwudu O, Dua K, Chellappan DK, Katavic PL, Collet TA. Recent update on anti-dengue drug discovery. Eur J Med Chem. 2019;176:431–55. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
