This is a preprint.
Mechanism of small heat shock protein client sequestration and induced polydispersity
- PMID: 39677757
- PMCID: PMC11642849
- DOI: 10.1101/2024.12.03.626640
Mechanism of small heat shock protein client sequestration and induced polydispersity
Update in
-
Mechanism of small heat shock protein client sequestration and induced polydispersity.Nat Commun. 2025 Apr 16;16(1):3635. doi: 10.1038/s41467-025-58964-3. Nat Commun. 2025. PMID: 40240363 Free PMC article.
Abstract
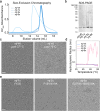
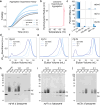
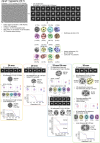
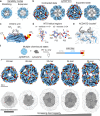
Small heat shock proteins (sHSPs) act as first responders during cellular stress by recognizing and sequestering destabilized proteins (clients), preventing their aggregation and facilitating downstream refolding or degradation1-3. This chaperone function is critically important to proteostasis, conserved across all kingdoms of life, and associated with various protein misfolding diseases in humans4,5. Mechanistic insights into how sHSPs sequester destabilized clients have been limited due to the extreme molecular plasticity and client-induced polydispersity of sHSP/client complexes6-8. Here, we present high-resolution cryo-EM structures of the sHSP from Methanocaldococcus jannaschii (mjHSP16.5) in both the apo-state and in an ensemble of client-bound states. The ensemble not only reveals key molecular mechanisms by which sHSPs respond to and sequester client proteins, but also provides insights into the cooperative nature of chaperone-client interactions. Engagement with destabilized client induces a polarization of stability across the mjHSP16.5 scaffold, proposed to facilitate higher-order assembly and enhance client sequestration capacity. Some higher-order sHSP oligomers appear to form through simple insertion of dimeric subunits into new geometrical features, while other higher-order states suggest multiple sHSP/client assembly pathways. Together, these results provide long-sought insights into the chaperone function of sHSPs and highlight the relationship between polydispersity and client sequestration under stress conditions.
Keywords: Chaperone; cryo-electron microscopy (cryo-EM); proteostasis; small heat shock protein (sHSP).
Conflict of interest statement
CONFLICT OF INTERESTS Authors declare no competing interests.
Figures







References
-
- Jakob U., Gaestel M., Engel K. & Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268, 1517–1520 (1993). - PubMed
-
- Caspers G.-J., Leunissen J. A. M. & de Jong W. W. The expanding small heat-shock protein family, and structure predictions of the conserved “α-crystallin domain”. J. Mol. Evol. 40, 238–248 (1995). - PubMed
-
- Kampinga H. H. & Garrido C. HSPBs: small proteins with big implications in human disease. Int J Biochem Cell Biol 44, 1706–10 (2012). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
