This is a preprint.
RNA-programmable cell type monitoring and manipulation in the human cortex with CellREADR
- PMID: 39677799
- PMCID: PMC11642864
- DOI: 10.1101/2024.12.03.626590
RNA-programmable cell type monitoring and manipulation in the human cortex with CellREADR
Update in
-
RNA-programmable cell-type monitoring and manipulation in the human cortex with CellREADR.Cell Rep. 2025 Aug 26;44(8):116037. doi: 10.1016/j.celrep.2025.116037. Epub 2025 Jul 22. Cell Rep. 2025. PMID: 40700016 Free PMC article.
Abstract
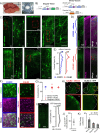
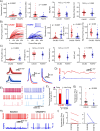
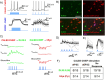
Reliable and systematic access to diverse cell types is necessary for understanding the organization, function, and pathophysiology of human neural circuits. Methods for targeting human neural populations are scarce and currently center on identifying transcriptional enhancers and engineering viral capsids. Here we demonstrate the utility of CellREADR, a programmable RNA sensor-effector technology that couples cellular RNA sensing to effector protein translation, for accessing, monitoring, and manipulating specific neuron types in human cortex, ex vivo. We designed CellREADRs to target two subpopulations, CALB2 GABAergic interneurons and FOXP2 glutamatergic projection neurons, then validated targeting specificity using histological, electrophysiological, and transcriptomic methods. CellREADR expression of channelrhodopsin and GCamp enabled the manipulation and monitoring of these populations in live cortical microcircuits. By demonstrating specific, reliable, and programmable experimental access to human neuronal subpopulations, our results highlight CellREADR's potential for studying neural circuits and treating brain disorders.
Keywords: Human neuroscience; PatchSeq; RNA sensor; calcium imaging; cellular access; cortex; excitatory neuron; interneuron; optogenetics; organotypic slice culture.
Conflict of interest statement
DECLARATION OF INTERESTS Z.J.H. has filed patents for CellREADR (US patent #: PCT/US22/79004 & PCT/US22/7900). Z.J.H is a co-founder of Doppler Bio. All other authors have no conflicts of interest to declare.
Figures


References
-
- Zeng H., and Sanes J.R. (2017). Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci. 18, 530–546. - PubMed
-
- Siletti K., Hodge R., Mossi Albiach A., Lee K.W., Ding S.-L., Hu L., Lönnerberg P., Bakken T., Casper T., Clark M., et al. (2023). Transcriptomic diversity of cell types across the adult human brain. Science 382, eadd7046. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
