CDC73 c.1155-3A>G is a pathogenic variant that causes aberrant splicing, disrupted parafibromin expression, and hyperparathyroidism-jaw tumor syndrome
- PMID: 39677927
- PMCID: PMC11646312
- DOI: 10.1093/jbmrpl/ziae149
CDC73 c.1155-3A>G is a pathogenic variant that causes aberrant splicing, disrupted parafibromin expression, and hyperparathyroidism-jaw tumor syndrome
Abstract
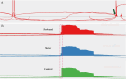
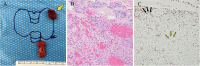
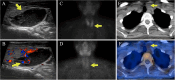
Germline and somatic pathogenic variants in the CDC73 gene, encoding the nuclear protein parafibromin, increase the risk for parathyroid carcinoma and cause hereditary primary hyperparathyroidism (PHPT) syndromes known as familial isolated hyperparathyroidism (FIHP) and hyperparathyroidism-jaw tumor syndrome (HPT-JT). The identification of pathogenic germline variants in PHPT-susceptibility genes can influence surgical planning for parathyroidectomy, guide screening for potential syndromic manifestations, and identify/exonerate at-risk family members. Numerous types of pathogenic germline variants have been described for CDC73-related conditions, including deletion, truncating, missense, and splice site mutations. Here, we report identification of a non-coding germline CDC73 variant (CDC73 c.1155-3A > G), previously categorized as a variant of uncertain significance (VUS), in a family with HPT-JT. This variant, found in two family members with PHPT, altered CDC73 splicing in peripheral blood cells and disrupted parafibromin immunostaining in associated parathyroid adenomas, strongly evidencing its pathogenicity. Sestamibi scintigraphy yielded nondiagnostic localization results for both patients' parathyroid adenomas, consistent with prior studies suggesting lower sensitivity for small or cystic lesions. Our findings demonstrate key aspects of CDC73-related disorders, highlight the diagnostic value of RNA testing, and exemplify the importance of obtaining a thorough, three-generational family history.
Keywords: Cdc73, aberrant splicing; HPT-JT; RNA sequencing; primary hyperparathyroidism.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

