Palladium-catalyzed aerobic homocoupling of aliphatic olefins to dienes: evidence for rate-limiting concerted metalation-deprotonation
- PMID: 39677937
- PMCID: PMC11638970
- DOI: 10.1039/d4sc06686c
Palladium-catalyzed aerobic homocoupling of aliphatic olefins to dienes: evidence for rate-limiting concerted metalation-deprotonation
Abstract
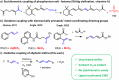
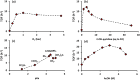
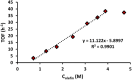
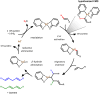
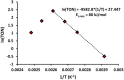
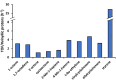
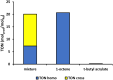
Palladium(ii)-catalyzed dehydrogenative coupling of aliphatic olefins would enable an efficient route to (conjugated) dienes, but remains scarcely investigated. Here, 2-hydroxypyridine (2-OH-pyridine) was found to be an effective ligand for Pd(ii) in the activation of vinylic C(sp2)-H bonds. While reoxidation of Pd(0) is challenging in many catalytic oxidations, one can avoid in this reaction that the reoxidation becomes rate-limiting, even under ambient O2 pressure, by working in coordinating solvents. Via kinetic studies the elementary steps governing this reaction were elucidated, resulting in enhanced performance (turnover frequency) of the Pd(ii)/2-OH-pyridine system. The diene product is formed via a consecutive activation of two olefins on the same Pd atom, followed by a β-hydride elimination. The first olefin activation, viz. the C-H activation, determines the overall reaction rate under these conditions. The catalytic complex was studied by ESI-MS and X-ray absorption spectroscopy, revealing that the coordination sphere of the working palladium complex contains two 2-OH-pyridine ligands.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
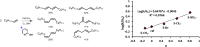


References
-
- Grub J. and Löser E., in Ullmann's Encyclopedia of Industrial Chemistry, Wiley, 2011, pp. 381–396
-
- Panten J. and Surburg H., in Ullmann's Encyclopedia of Industrial Chemistry, Wiley, 2015, pp. 1–55
-
- Metcalf R. and Horowitz A., in Ullmann's Encyclopedia of Industrial Chemistry, Wiley, 2014, pp. 1–94
-
- Breitmaier E., Terpenes: Flavors, Fragrances, Pharmaca, Pheromones, Wiley, 2006
LinkOut - more resources
Full Text Sources

