Long term efficacy and safety profile of dexamethasone intravitreal implant in retinal vein occlusions: a systematic review
- PMID: 39678032
- PMCID: PMC11641122
- DOI: 10.3389/fmed.2024.1454591
Long term efficacy and safety profile of dexamethasone intravitreal implant in retinal vein occlusions: a systematic review
Abstract
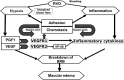
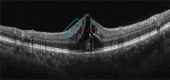
Background/objective: Retinal vein occlusion (RVO) is a common, sight-threatening vascular disorder affecting individuals of all ages, with incidence increasing with age. Due to its complex, multifactorial nature, treating RVO remains a clinical challenge. Currently, treatment strategies include laser photocoagulation (especially for branch RVO), anti-VEGF therapies, and intravitreal corticosteroids. This systematic review (without meta-analysis) aimed to update the evidence on the efficacy and safety of the sustained-release intravitreal dexamethasone implant (DEX-i) in managing macular edema (ME) secondary to central and branch RVO.
Methods: A systematic review was conducted to assess current literature on DEX-i for ME secondary to RVO. Relevant studies were analyzed for outcomes related to visual acuity, retinal thickness, and the safety profile of DEX-i in RVO treatment.
Results: Evidence indicates that DEX-i substantially improves best-corrected visual acuity (BCVA) and reduces central retinal thickness (CRT) in ME associated with both branch and central RVO, demonstrating rapid and sustained effects. Common adverse events associated with DEX-i included manageable complications, such as medically controlled intraocular pressure elevation and progression of cataracts.
Conclusion: DEX-i offers effective and sustained improvements in both visual and anatomical outcomes for patients with ME secondary to RVO. Individualized treatment selection is essential to optimize patient outcomes. Future directions include identifying predictive biomarkers and adopting patient-centered approaches based on individual clinical characteristics, which may enhance treatment success in RVO.
Keywords: branch retinal vein occlusion; central retinal vein occlusion; dexamethasone intravitreal implant; macular edema; retinal vein occlusion.
Copyright © 2024 Carnevali, Bacherini, Metrangolo, Chiosi, Viggiano, Astarita, Gallinaro and Bonfiglio.
Conflict of interest statement
This study received funding from AbbVie. The funder had the following involvement in the study: participation in the design of the manuscript and review of the data. CA and VG were AbbVie employees and may own AbbVie stocks/options. CM was consultant for Novartis, Bayer, and Omega Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Rogers S, McIntosh R, Cheung N, Lim L, Wang J, Mitchell P, et al. International eye disease consortium. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. (2010) 117(2):313–9.e1. 10.1016/j.ophtha.2009.07.017 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

