Traversing the epigenetic landscape: DNA methylation from retina to brain in development and disease
- PMID: 39678047
- PMCID: PMC11637887
- DOI: 10.3389/fncel.2024.1499719
Traversing the epigenetic landscape: DNA methylation from retina to brain in development and disease
Abstract
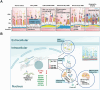
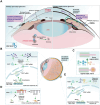
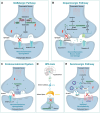
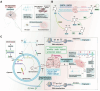
DNA methylation plays a crucial role in development, aging, degeneration of various tissues and dedifferentiated cells. This review explores the multifaceted impact of DNA methylation on the retina and brain during development and pathological processes. First, we investigate the role of DNA methylation in retinal development, and then focus on retinal diseases, detailing the changes in DNA methylation patterns in diseases such as diabetic retinopathy (DR), age-related macular degeneration (AMD), and glaucoma. Since the retina is considered an extension of the brain, its unique structure allows it to exhibit similar immune response mechanisms to the brain. We further extend our exploration from the retina to the brain, examining the role of DNA methylation in brain development and its associated diseases, such as Alzheimer's disease (AD) and Huntington's disease (HD) to better understand the mechanistic links between retinal and brain diseases, and explore the possibility of communication between the visual system and the central nervous system (CNS) from an epigenetic perspective. Additionally, we discuss neurodevelopmental brain diseases, including schizophrenia (SZ), autism spectrum disorder (ASD), and intellectual disability (ID), focus on how DNA methylation affects neuronal development, synaptic plasticity, and cognitive function, providing insights into the molecular mechanisms underlying neurodevelopmental disorders.
Keywords: Alzheimer’s disease and Huntington’s disease; DNA methylation and DNA demethylation; age-related macular degeneration; autism spectrum disorder; diabetic retinopathy; glaucoma; intellectual disability; schizophrenia.
Copyright © 2024 Xu, Fu, Qin and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abdolmaleky H. M., Pajouhanfar S., Faghankhani M., Joghataei M. T., Mostafavi A., Thiagalingam S. (2015). Antipsychotic drugs attenuate aberrant DNA methylation of DTNBP1 (dysbindin) promoter in saliva and post-mortem brain of patients with schizophrenia and psychotic bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168, 687–696. doi: 10.1002/ajmg.b.32361, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

