Leveraging high-throughput analytics and automation to rapidly develop high-concentration mAb formulations: integrated excipient compatibility and viscosity screening
- PMID: 39678259
- PMCID: PMC11646310
- DOI: 10.1093/abt/tbae028
Leveraging high-throughput analytics and automation to rapidly develop high-concentration mAb formulations: integrated excipient compatibility and viscosity screening
Abstract
Background: Formulation screening is essential to experimentally balance stability and viscosity in high-concentration mAb formulations. We developed a high-throughput approach with automated sample preparation and analytical workflows to enable the integrated assessment of excipient compatibility and viscosity of mAb formulations.
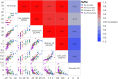
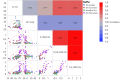
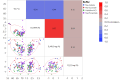
Methods: Ninety-six formulations of a trastuzumab biosimilar were screened by combining 8 types of excipient modifiers with 4 types of buffers across a pH range of 4.5 to 7.5. Key stability risks, including high molecular weight (HMW) aggregation and fragmentation, were thoroughly assessed along with viscosity at high concentrations. Additionally, several biophysical parameters were evaluated for their ability to predict stability or viscosity outcomes. Multiple linear regression was applied to fit the data and identify key factors.
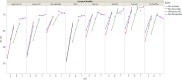
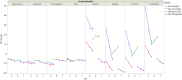
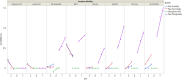
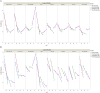
Results: The optimal pH range for the trastuzumab biosimilar was found to be 5.0 to 6.5, based on opposing pH dependencies for stability and viscosity. Buffer type had a minor effect on viscosity and fragmentation but played a significant role in influencing HMW aggregates, with Na-acetate and histidine-HCl being the best candidates. The impact of excipient modifiers on viscosity, HMW, and fragmentation depended on both pH and buffer type, showing strong interactions among factors. Arginine-HCl and lysine-HCl effectively lowered viscosity of the trastuzumab biosimilar at pH levels above 6.0, while glycine formulations were more effective at reducing viscosity below pH 6.0. Histidine-HCl, arginine-HCl, and lysine-HCl lowered the risk of HMW aggregation, whereas formulations containing Na-phosphate or NaCl showed higher HMW aggregation. Formulations with arginine-HCl, lysine-HCl, and NaCl demonstrated a rapid increase in fragmentation at pH levels below 5.0, while Na-aspartate formulations showed increased fragmentation at pH levels above 6.5.
Conclusion: Hence, it is important to optimize the levels of each chosen excipient in the formulation study to balance their benefits against potential incompatibilities. This study serves as a foundation for identifying high-concentration antibody formulations using a high-throughput approach, where minimal materials are required, and optimized formulation design spaces can be quickly identified.
Keywords: automated buffer exchange and ultrafiltration; colloidal interaction; excipient compatibility; high-concentration antibody formulation; melting temperature; multiple linear regression; stability; viscosity.
© The Author(s) 2024. Published by Oxford University Press on behalf of Antibody Therapeutics.
Conflict of interest statement
The authors would like to disclose that funding for the study is provided by Catalent Pharma Solutions. At the time of study, all authors were employees of Catalent Pharma Solutions and may hold Catalent’s stock. Yunsong Li is a guest editor of Antibody Therapeutics and was blinded from reviewing or making decisions for this manuscript.
Figures


References
LinkOut - more resources
Full Text Sources
