This is a preprint.
Myosin-dependent short actin filaments contribute to peripheral widening in developing stereocilia
- PMID: 39678325
- PMCID: PMC11643313
- DOI: 10.21203/rs.3.rs-5448262/v1
Myosin-dependent short actin filaments contribute to peripheral widening in developing stereocilia
Update in
-
Myosin-dependent short actin filaments contribute to peripheral widening in developing stereocilia.Nat Commun. 2025 Jul 1;16(1):5835. doi: 10.1038/s41467-025-60976-y. Nat Commun. 2025. PMID: 40593742 Free PMC article.
Abstract
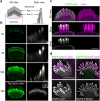
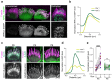
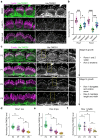
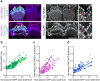
In the auditory and vestibular systems, stereocilia are actin-based protrusions that convert mechanical stimuli into electrical signals. During development, stereocilia elongate and widen by adding filamentous actin (F-actin), attaining their mature shape necessary for mechanosensitive function. Myosin motors, including MYO3A/B and MYO15A, are required for normal stereocilia growth, but the regulation of actin and the impact of myosins on actin assembly remain unclear. We focused on stereocilia widening, which requires the addition of new filaments to the bundle of linear F-actin comprising the initial stereocilia core. Our findings revealed that newly expressed actin incorporates at the stereocilia tip first, then along the shaft to promote stereocilia widening. The newly incorporated F-actin surrounded the existing F-actin core, suggesting that the core is stable once formed, with additional actin adding only to the periphery. To better understand the nature of incorporating actin, we used several probes to detect globular (G-) actin, F-actin barbed ends, and F-actin pointed ends. While F-actin core filaments are parallel and thought to present only barbed ends at stereocilia tips, we also detected F-actin pointed ends, indicating a previously undetected population of short actin filaments. Overexpression of actin resulted in abundant F-actin pointed and barbed ends along the periphery of the stereocilia shaft, suggesting that short actin filaments contribute to stereocilia widening. Short actin filament levels correlated with the levels of MYO3A/B and MYO15A at stereocilia tips, suggesting these myosins generate or stabilize short actin filaments essential for stereocilia widening and elongation.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

