This is a preprint.
Albumin orchestrates a natural host defense mechanism against mucormycosis
- PMID: 39678331
- PMCID: PMC11643317
- DOI: 10.21203/rs.3.rs-5441197/v1
Albumin orchestrates a natural host defense mechanism against mucormycosis
Abstract
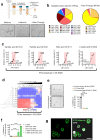
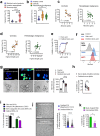
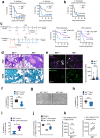
Mucormycosis is an emerging, life-threatening human infection caused by fungi of the order Mucorales. Metabolic disorders uniquely predispose an ever-expanding group of patients to mucormycosis via poorly understood mechanisms. Therefore, it is highly likely that uncharacterized host metabolic effectors confer protective immunity against mucormycosis. Here, we uncover a master regulatory role of albumin in host defense against Mucorales through the modulation of the fungal pathogenicity program. Our initial studies identified severe hypoalbuminemia as a prominent metabolic abnormality and a biomarker of poor outcome in independent cohorts of mucormycosis patients. Strikingly, we found that purified albumin selectively inhibits Mucorales growth among a range of human pathogens, and albumin-deficient mice display susceptibility specifically to mucormycosis. The antifungal activity of albumin is mediated by the release of bound free fatty acids (FFAs). Importantly, albumin prevents FFA oxidation, which results in loss of their antifungal properties. A high degree of FFA oxidation is found in the sera of patients with mucormycosis. Physiologically, albumin-bound FFAs blocks the expression of the mycotoxin mucoricin and renders Mucorales avirulent in vivo. Overall, we discovered a novel host defense mechanism that directs the pathogen to suppress its growth and the expression of virulence factors in response to unfavorable metabolic cues regulated by albumin. These findings have major implications for the pathogenesis and management of mucormycosis.
Keywords: Albumin; Free fatty acids; Mucorales; Rhizopus; fungi; host defense; mucoricin; mucormycosis; serum.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

