Efficacy and safety of SQ house dust mite sublingual immunotherapy-tablet (12 SQ-HDM) in children with allergic rhinitis/rhinoconjunctivitis with or without asthma (MT-12): a randomised, double-blind, placebo-controlled, phase III trial
- PMID: 39678704
- PMCID: PMC11638617
- DOI: 10.1016/j.lanepe.2024.101136
Efficacy and safety of SQ house dust mite sublingual immunotherapy-tablet (12 SQ-HDM) in children with allergic rhinitis/rhinoconjunctivitis with or without asthma (MT-12): a randomised, double-blind, placebo-controlled, phase III trial
Abstract
Background: Allergic rhinitis/rhinoconjunctivitis (AR/C) induced by house dust mites (HDM) often begins in childhood and negatively impacts a child's quality of life. The daily burden can be further compounded by comorbid asthma. Allergen immunotherapy is the only available treatment targeting the underlying cause of allergic disease. Efficacy and safety of the SQ HDM sublingual immunotherapy (SLIT)-tablet has been demonstrated in adults and adolescents with HDM AR/C with or without asthma, but data are lacking for younger children.
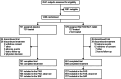
Methods: Phase III, randomised, double-blind, placebo-controlled trial in younger children (5-11 years) with HDM AR/C with or without asthma. Eligible subjects were randomised 1:1 to SQ HDM SLIT-tablet or placebo for ∼1 year and had free access to AR/C symptom-relieving medications. The primary outcome was the total combined rhinitis score (TCRS) during the final 8 weeks of the treatment period (∼1 year). Secondary outcomes included the rhinitis daily symptom score (DSS) and medication score (DMS), the rhinoconjunctivitis total combined score (TCS), and the Paediatric Rhinoconjunctivitis Quality of Life Questionnaire (PRQLQ) score. Efficacy analyses were conducted on the full analysis set (observed cases). Asthma-related outcomes were also explored. The trial was registered on ClinicalTrials.gov: NCT04145219 and EudraCT: 2019-000560-22.
Findings: A total of 1460 subjects were randomised to SQ HDM SLIT-tablet (n = 729) or placebo (n = 731). The primary outcome, TCRS, was statistically significantly different for SQ HDM SLIT-tablet (n = 693) versus placebo (n = 706), with an absolute difference of 1.0 (95% CI: 0.5, 1.4; p < 0.0001) corresponding to a relative reduction of 22.0% (95% CI: 12.0, 31.1). Key secondary outcomes (DSS, DMS, TCS, PRQLQ) showed statistically significant reductions in symptoms and medication use, and improved disease-related quality of life for SQ HDM SLIT-tablet versus placebo. Improvements in asthma symptoms and reduced asthma medication use indicated an additional effect of SQ HDM-SLIT tablet versus placebo. The SQ HDM SLIT-tablet showed a higher event rate for treatment-related adverse events (AEs) than placebo. Most events were of mild or moderate severity and few subjects discontinued due to AEs (2.5%).
Interpretation: The trial confirmed the efficacy and safety of the SQ HDM SLIT-tablet for treating HDM AR/C in younger children (5-11 years) with or without asthma. The safety profile supports daily self-administration of the SQ HDM SLIT-tablet in children.
Funding: ALK-Abellό, Hørsholm, Denmark.
Keywords: Allergen immunotherapy; Allergic rhinitis; House dust mite; Paediatric; SLIT-tablet.
© 2024 The Author(s).
Conflict of interest statement
AS has received personal fees/clinical study fees from ALK-Abelló. Unpaid board member for German paediatric scientific societies (Gesellschaft für Pädiatrische Pneumologie [GPP] and Westdt. AG für pädiatrische Pneumologie und Allergologie [WAPPA]; unpaid co-author on scientific guidelines. DC has received fees for oral presentations from ALK-Abelló, Stallergenes Greer, AstraZeneca, GlaxoSmithKline, and Sanofi-Genzyme. Scientific board member for ALK-Abelló, Stallergenes Greer, AstraZeneca, and Sanofi-Genzyme. HN is an employee of ALK-Abelló. SN has received fees for oral presentations from Stallergenes Greer, TEVA. Chie, Berlin–Chemie, and Chiesi. JM was a clinical investigator in clinical studies MT-12, MT-18, and TT-06 sponsored by ALK-Abelló, and YOBI-SL79.22 sponsored by Stallergenes. MHF-S is an employee of ALK-Abelló. ASØ was an employee of ALK-Abelló when the work was conducted, but has since moved to Novo Nordisk. AE has received lecture fees from ALK-Abelló. RG has conducted research for ALK-Abelló, AstraZeneca, GSK, DBV, Regeneron, Moderna, Novartis, and Sanofi, and has received consultancy fees from ALK-Abelló, Regeneron, Bausch Lomb, and Sanofi-Genzyme; received lecture fees from Bausch Lomb, and Novartis. OP reports grants from ALK-Abelló, Denmark, during the conduct of the study; furthermore, he reports grants and/or personal fees and/or travel support from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Laboratorios LETI/LETI Pharma, GSK, ROXALL Medizin, Novartis, Sanofi-Aventis and Sanofi-Genzyme, Med Update Europe GmbH, streamedup! GmbH, Pohl-Boskamp, Inmunotek S.L., John Wiley and Sons/AS, Paul-Martini-Stiftung (PMS), Regeneron Pharmaceuticals Inc., RG Aerztefortbildung, Institut für Disease Management, Springer GmbH, AstraZeneca, IQVIA Commercial, Ingress Health, Wort&Bild Verlag, Verlag ME, Procter&Gamble, ALTAMIRA, Meinhardt Congress GmbH, Deutsche Forschungsgemeinschaft, Thieme, Deutsche AllergieLiga e.V., AeDA, Alfried-Krupp Krankenhaus, Red Maple Trials Inc., Königlich Dänisches Generalkonsulat, Medizinische Hochschule Hannover, ECM Expo & Conference Management GmbH, Technical University Dresden, Lilly, Japanese Society of Allergy, Forum für Medizinische Fortbildung, Dustri-Verlag, Pneumolive, ASIT Biotech, LOFARMA, Almirall, Paul-Ehrlich-Institut, all outside the submitted work; and he is member of EAACI Excom, member of ext. board of directors DGAKI; coordinator, main- or co-author of different position papers and guidelines in rhinology, allergology and allergen-immunotherapy; he is associate editor (AE) of Allergy and Clinical Translational Allergy.
Figures


References
-
- Bousquet J., Khaltaev N., Cruz A.A., et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63:8–160. - PubMed
-
- Asher M.I., Montefort S., Björkstén B., et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. - PubMed
-
- Frati F., Scurati S., Dell’Albani I., Puccinelli P., Incorvaia C., Passalacqua G. Evaluation of house dust mite allergy in real life: patients’ characteristics and satisfaction with treatment. Eur Ann Allergy Clin Immunol. 2014;46:17–21. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

