Impact of Sodium-Glucose Co-Transporter-2 Inhibitors on Exercise-Induced Pulmonary Hypertension
- PMID: 39678732
- PMCID: PMC11646329
- DOI: 10.1002/pul2.70026
Impact of Sodium-Glucose Co-Transporter-2 Inhibitors on Exercise-Induced Pulmonary Hypertension
Abstract
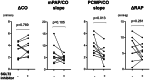
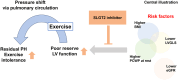
Patients with borderline pulmonary hypertension (PH) often experience shortness of breath or exacerbation of PH during exercise, known as exercise-induced PH. However, the pathogenesis of exercise-induced post-capillary PH (post-EIPH) and its treatment strategies remain unclear. Recent guidelines and consensus documents have highlighted the benefits of sodium-glucose cotransporter-2 (SGLT2) inhibitors in heart failure and chronic kidney disease (CKD). This study aimed to investigate the effects of SGLT2 inhibitors in patients with post-EIPH and CKD. This single-center prospective cohort study enroled 10 patients with CKD (age, 68 years; female, 60%) who exhibited post-EIPH between 1 July 2022 and 31 December 2023. Post-EIPH was defined as a pulmonary capillary wedge pressure (PCWP)/cardiac output (CO) slope > 2 and peak PCWP during exercise ≥ 25 mmHg measured by catheterization. The patients received SGLT2 inhibitor treatment for 6 months. At rest, patients with post-EIPH had borderline-PH (21.5 ± 1.8 mmHg), with preserved left and right ventricular function. SGLT2 inhibitors treatment significantly reduced the PCWP/CO slope during exercise (3.9 ± 1.2 vs. 2.4 ± 1.2 mmHg/L/min, p = 0.013) and improved the 6-min walking distance (489.9 ± 80.2 vs. 568.3 ± 91.9 m, p = 0.014). Magnetic resonance imaging revealed a lower left ventricular global longitudinal strain in patients with post-EIPH, which was increased by SGLT2 inhibitor treatment (-13.8 ± 2.0 vs. -17.3 ± 2.0%, p = 0.003). SGLT2 treatment inhibitors mitigated post-EIPH hemodynamic abnormalities and exercise intolerance, suggesting their potential as its therapeutic option.
Keywords: MRI; SGLT2 inhibitor; exercise induced pulmonary hypertension; right heart catheterization during exercise.
© 2024 The Author(s). Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
Y.S. has received grant support from Takeda Pharmaceutical, Abbott, and Boston Scientific and lecture fees from Daiichi Sankyo and Bristol Myers Squibb. The remaining authors have nothing to disclose.
Figures

References
-
- Kovacs G., Avian A., Tscherner M., et al., “Characterization of Patients With Borderline Pulmonary Arterial Pressure,” Chest 146 (2014): 1486–1493. - PubMed
-
- Humbert M., Kovacs G., Hoeper M. M., et al., “ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension,” European Heart Journal 2022, no. 43 (2022): 3618–3731. - PubMed
-
- Pieske B., Tschöpe C., de Boer R. A., et al., “How to Diagnose Heart Failure With Preserved Ejection Fraction: The HFA‐PEFF Diagnostic Algorithm: A Consensus Recommendation From the Heart Failure Association (HFA) of the European Society of Cardiology (ESC),” European Journal of Heart Failure 22 (2020): 391–412. - PubMed
-
- Kidney Disease: Improving Global Outcomes Diabetes Work G , “KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease,” Kidney International 102 (2022): S1–S127. - PubMed
LinkOut - more resources
Full Text Sources

