Enhancing the radiosensitivity of colorectal cancer cells by reducing spermine synthase through promoting autophagy and DNA damage
- PMID: 39678812
- PMCID: PMC11577379
- DOI: 10.4251/wjgo.v16.i12.4716
Enhancing the radiosensitivity of colorectal cancer cells by reducing spermine synthase through promoting autophagy and DNA damage
Abstract
Background: Colorectal cancer (CRC), the third most common cancer worldwide, has increasingly detrimental effects on human health. Radiotherapy resistance diminishes treatment efficacy. Studies suggest that spermine synthase (SMS) may serve as a potential target to enhance the radiosensitivity.
Aim: To investigate the association between SMS and radiosensitivity in CRC cells, along with a detailed elucidation of the underlying mechanisms.
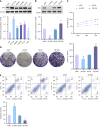
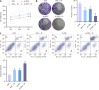
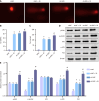
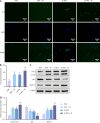
Methods: Western blot was adopted to assess SMS expression in normal colonic epithelial cells and CRC cell lines. HCT116 cells were transfected with control/SMS-specific shRNA or control/pcDNA3.1-SMS plasmids. Assessments included cell viability, colony formation, and apoptosis via MTT assays, colony formation assays, and flow cytometry. Radiosensitivity was studied in SMS-specific shRNA-transfected HCT116 cells post-4 Gy radiation, evaluating cell viability, colony formation, apoptosis, DNA damage (comet assays), autophagy (immunofluorescence), and mammalian target of rapamycin (mTOR) pathway protein expression (western blot).
Results: Significant up-regulation of SMS expression levels was observed in the CRC cell lines. Upon down-regulation of SMS expression, cellular viability and colony-forming ability were markedly suppressed, concomitant with a notable increase in apoptotic indices. Furthermore, attenuation of SMS expression significantly augmented the sensitivity of HCT116 cells to radiation therapy, evidenced by a pronounced elevation in levels of cellular DNA damage and autophagy. Importantly, down-regulation of SMS corresponded with a marked reduction in the expression levels of proteins associated with the mTOR signaling pathway.
Conclusion: Knocking down SMS attenuates the mTOR signaling pathway, thereby promoting cellular autophagy and DNA damage to enhance the radiosensitivity of CRC cells.
Keywords: Autophagy; Colorectal cancer; DNA damage; Radiosensitivity; Spermine synthase.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no competing interests.
Figures

Similar articles
-
MicroRNA-126 Inhibit Viability of Colorectal Cancer Cell by Repressing mTOR Induced Apoptosis and Autophagy.Onco Targets Ther. 2020 Mar 24;13:2459-2468. doi: 10.2147/OTT.S238348. eCollection 2020. Onco Targets Ther. 2020. Retraction in: Onco Targets Ther. 2024 Feb 20;17:129-130. doi: 10.2147/OTT.S464377. PMID: 32273718 Free PMC article. Retracted.
-
Andrographolide enhanced radiosensitivity by downregulating glycolysis via the inhibition of the PI3K-Akt-mTOR signaling pathway in HCT116 colorectal cancer cells.J Int Med Res. 2020 Aug;48(8):300060520946169. doi: 10.1177/0300060520946169. J Int Med Res. 2020. PMID: 32787737 Free PMC article.
-
Gambogic acid enhances the radiosensitivity of human esophageal cancer cells by inducing reactive oxygen species via targeting Akt/mTOR pathway.Tumour Biol. 2016 Feb;37(2):1853-62. doi: 10.1007/s13277-015-3974-1. Epub 2015 Aug 30. Tumour Biol. 2016. PMID: 26318432
-
[Down regulation of multidrug resistance-associated protein 4 expression by RNA interference enhances radiosensitivity of colorectal carcinoma cell lines in vitro].Zhonghua Wei Chang Wai Ke Za Zhi. 2012 Jan;15(1):67-71. Zhonghua Wei Chang Wai Ke Za Zhi. 2012. PMID: 22287356 Chinese.
-
TOPK Inhibition Enhances the Sensitivity of Colorectal Cancer Cells to Radiotherapy by Reducing the DNA Damage Response.Curr Med Sci. 2024 Jun;44(3):545-553. doi: 10.1007/s11596-024-2884-0. Epub 2024 Jun 20. Curr Med Sci. 2024. PMID: 38900386
Cited by
-
PI3K/AKT/mTOR Targeting in Colorectal Cancer Radiotherapy: A Systematic Review.J Gastrointest Cancer. 2025 Jan 24;56(1):52. doi: 10.1007/s12029-024-01160-1. J Gastrointest Cancer. 2025. PMID: 39849185
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233–254. - PubMed
-
- Kędzia-Berut R, Berut M, Włodarczyk M, Włodarczyk J, Dziki Ł, Dziki A, Mik M. Colorectal Cancer: Is it Still a Disease of the Elderly? Pol Przegl Chir. 2023;96:41–45. - PubMed
-
- Tessmann JW, Rocha MR, Morgado-Díaz JA. Mechanisms of radioresistance and the underlying signaling pathways in colorectal cancer cells. J Cell Biochem. 2023;124:31–45. - PubMed
-
- Wang C, Yuan M, Gao Y, Hou R, Song D, Feng Y. Changes in Tumor Immune Microenvironment after Radiotherapy Resistance in Colorectal Cancer: A Narrative Review. Oncol Res Treat. 2023;46:177–191. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

