Investigation of Antibacterial Coatings Based on Chitosan/Polyacrylic Acid/Chlorhexidine for Orthopedic Implants
- PMID: 39679059
- PMCID: PMC11638784
- DOI: 10.1021/acspolymersau.4c00049
Investigation of Antibacterial Coatings Based on Chitosan/Polyacrylic Acid/Chlorhexidine for Orthopedic Implants
Abstract
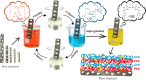
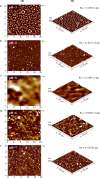
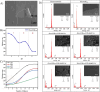
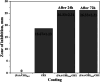
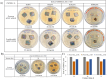
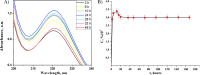
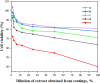
Antibacterial coatings on model silicon wafers and implants, based on chitosan (CHI), poly(acrylic acid) (PAA), and the antibacterial agent chlorhexidine digluconate (CHX), were obtained using a layer-by-layer assembly method. The surface roughness and 2D and 3D images of the surfaces of CHI/PAA/CHX coatings obtained from different pH assemblies were investigated by atomic force microscopy, revealing that pH 6 enabled optimal inclusion of CHX in the multilayer film. The structure and elemental composition before and after implementation of CHX into the coating were investigated via scanning electron microscopy and energy-dispersive X-ray spectroscopy. The obtained films exhibited antimicrobial efficacy against Staphylococcus aureus and Staphylococcus epidermidis. The effects of CHX concentration and duration of contact with the coating on bacterial activity were investigated, and the quantitative release of CHX from coated implants in phosphate buffer was determined as a function of the incubation time. The biocompatibility of the PAA/CHI/CHX coatings was investigated using human mononuclear cells (HMNCs) and quantified using an MTT assay. HMNCs demonstrated high viability in eluted solutions obtained from implants coated with PAA/CHI/CHX (0.025%) and PAA/CHI/CHX (0.0125%), while the extract of implants coated with PAA/CHI/CHX (0.05%) induced slight cytotoxicity.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Udduttula A.; Jakubovics N.; Khan I.; Pontiroli L.; Rankin K. S.; Gentile P.; Ferreira A. M. Layer-by-Layer Coatings of Collagen–Hyaluronic Acid Loaded with an Antibacterial Manuka Honey Bioactive Compound to Fight Metallic Implant Infections. ACS Appl. Mater. Interfaces 2023, 15 (50), 58119–58135. 10.1021/acsami.3c11910. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
