Synthesis and Characterization of α,ω-End Orthogonally Functionalizable Glycopolymers from Native Glycans
- PMID: 39679285
- PMCID: PMC11636928
- DOI: 10.1039/d4py00191e
Synthesis and Characterization of α,ω-End Orthogonally Functionalizable Glycopolymers from Native Glycans
Abstract
Glycopolymers have been employed as biomimetic glycoconjugates in both biological and biomedical research and applications. Among them, chain-end functionalized glycopolymers are very often explored for protein modification, microarray, biosensor, bioprobe and other applications. Herein, we report a straightforward synthesis of α,ω-end orthogonally functionalizable glycopolymers. Specifically, glycopolymers with an alkyne or azide group at one end and an O-cyanate on the other end were synthesized via cyanoxyl-mediated free-radical polymerization from native glycans without protection and deprotection. The alkyne chain-end can react with azide-containing molecules via click chemistry. The azide chain-end can react with alkyne-containing molecules via click chemistry or copper free click chemistry. On the other hand, O-cyanate can react with an amine group via isourea bond, affording a site-specific bioconjugation as well. Furthermore, chain-end heterofunctionalizations of the glycopolymers were demonstrated via sequential or one-pot click chemistry and isourea bond formation, respectively. Finally, end-to-end dimerization of the glycopolymers was demonstrated via chain-end click chemistry. These α,ω-end orthogonally functionalizable glycopolymers will be useful in many biological and biomedical research applications.
Keywords: N-glycan; click chemistry; cyanoxyl-mediated free radical polymerization; glycopolymer; glycosylamine; sialylglycan.
Conflict of interest statement
Conflict of Interest The authors declare no competing financial interests.
Figures




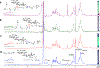
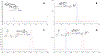
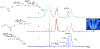
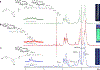




References
-
- Thalji MR,; Amin A, Kwok I, Chong F, Glycopolymer - Based Materials : Synthesis, Properties,; Springer International Publishing, 2022; Vol. 380.
-
- Miura Y, Design and Synthesis of Well-Defined Glycopolymers for the Control of Biological Functionalities. Polym J 2012, 44, 679–689.
-
- Mancini RJ, Lee J, Maynard HD, Trehalose Glycopolymers for Stabilization of Protein Conjugates to Environmental Stressors. J Am Chem Soc 2012, 134, 8474–8479. - PubMed
-
- Zhang H, Weingart J, Gruzdys V, Sun XL, Synthesis of an End-to-End Protein-Glycopolymer Conjugate via Bio-Orthogonal Chemistry. ACS Macro Lett 2016, 5, 73–77. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
