Inhibition and evasion of neutrophil microbicidal responses by Legionella longbeachae
- PMID: 39679679
- PMCID: PMC11796426
- DOI: 10.1128/mbio.03274-24
Inhibition and evasion of neutrophil microbicidal responses by Legionella longbeachae
Abstract
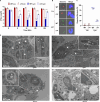
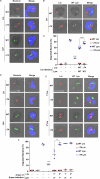
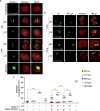
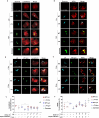
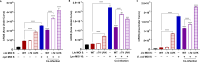
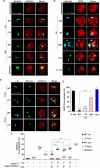
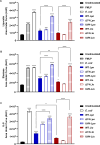
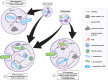
Legionella species evade degradation and proliferate within alveolar macrophages as an essential step for the manifestation of disease. However, most intracellular bacterial pathogens are restricted in neutrophils, which are the first line of innate immune defense against invading pathogens. Bacterial degradation within neutrophils is mediated by the fusion of microbicidal granules to pathogen-containing phagosomes and the generation of reactive oxygen species (ROS) by the phagocyte NADPH oxidase complex. Here, we show that human neutrophils fail to trigger microbicidal processes and, consequently, fail to restrict L. longbeachae. In addition, neutrophils infected with L. longbeachae fail to undergo a robust pro-inflammatory response, such as degranulation and IL-8 production. Here, we identify three strategies employed by L. longbeachae for evading restriction by neutrophils and inhibiting the neutrophil microbicidal response to other bacteria co-inhabiting in the same cell. First, L. longbeachae excludes the cytosolic and membrane-bound subunits of the phagocyte NADPH oxidase complex from its phagosomal membrane independent of the type 4 secretion system (T4SS). Consequently, infected neutrophils fail to generate robust ROS in response to L. longbeachae. Second, L. longbeachae impedes the fusion of azurophilic granules to its phagosome and the phagosomes of bacteria co-inhabiting the same cell through T4SS-independent mechanisms. Third, L. longbeachae protects phagosomes of co-inhabiting bacteria from degradation by ROS through a trans-acting T4SS-dependent mechanism. Collectively, we conclude that L. longbeachae evades restriction by human neutrophils via T4SS-independent mechanisms and utilizes trans-acting T4SS-dependent mechanisms for inhibition of neutrophil ROS generation throughout the cell cytosol.
Importance: Legionella longbeachae is commonly found in soil environments where it interacts with a wide variety of protist hosts and microbial competitors. Upon transmission to humans, L. longbeachae invades and replicates within alveolar macrophages, leading to the manifestation of pneumonia. In addition to alveolar macrophages, neutrophils are abundant immune cells acting as the first line of defense against invading pathogens. While most intracellular bacterial species are killed and degraded by neutrophils, we show that L. longbeachae evades degradation. The pathogen impairs the major neutrophils' microbicidal processes, including the fusion of microbicidal granules to the pathogen-containing vacuole. By inhibiting of assembly of the phagocyte NADPH oxidase complex, the pathogen blocks neutrophils from generating microbicide reactive oxygen species. Overall, L. longbeachae employs unique virulence strategies to evade the major microbicidal processes of neutrophils.
Keywords: NADPH oxidase; ROS; azurphilic granules; granules; lysosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cazalet C, Gomez-Valero L, Rusniok C, Lomma M, Dervins-Ravault D, Newton HJ, Sansom FM, Jarraud S, Zidane N, Ma L, Bouchier C, Etienne J, Hartland EL, Buchrieser C. 2010. Analysis of the Legionella longbeachae genome and transcriptome uncovers unique strategies to cause Legionnaires’ disease. PLoS Genet 6:e1000851. doi: 10.1371/journal.pgen.1000851 - DOI - PMC - PubMed
-
- Dolinsky S, Haneburger I, Cichy A, Hannemann M, Itzen A, Hilbi H. 2014. The Legionella longbeachae Icm/Dot substrate SidC selectively binds phosphatidylinositol 4-phosphate with nanomolar affinity and promotes pathogen vacuole-endoplasmic reticulum interactions. Infect Immun 82:4021–4033. doi: 10.1128/IAI.01685-14 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

