Ca2+- and cGAMP-Contained Semiconducting Polymer Nanomessengers for Radiodynamic-Activated Calcium Overload and Immunotherapy
- PMID: 39679909
- PMCID: PMC11809400
- DOI: 10.1002/advs.202411739
Ca2+- and cGAMP-Contained Semiconducting Polymer Nanomessengers for Radiodynamic-Activated Calcium Overload and Immunotherapy
Abstract
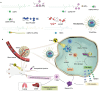
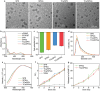
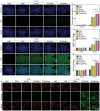
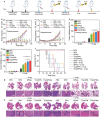
Various second messengers exert some vital actions in biological systems, including cancer therapy, but the therapeutic efficacy is often need to be improved. A semiconducting polymer nanomessenger (TCa/SPN/a) consisting of two second messengers, calcium ion (Ca2+) and cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) for metastatic breast cancer therapy, is reported here. Such a TCa/SPN/a is constructed to exhibit X-ray response for the activatable delivery of mitochondria-targeting Ca compound and cGAMP as stimulator of interferon genes (STING) agonist. With X-ray irradiation, TCa/SPN/a could generate singlet oxygen (1O2) via radiodynamic effect for ablating solid tumors and improving the tumor immunogenicity by inducing immunogenic cell death (ICD). Furthermore, the released mitochondria-targeting Ca compounds show a high binging effect on mitochondria and cause reactive oxygen species (ROS) generation and mitochondria damage via calcium overload, while cGAMP boosts immunological effect through activating STING pathway. In this way, TCa/SPN/a enables a radiodynamic-activated calcium overload and immunotherapy to obviously inhibit the growths of bilateral tumors and also abolish tumor metastasis in metastatic breast cancer mouse models. This article should demonstrate the first smart dual-functional nanotherapeutic containing two second messengers for precise and specific cancer therapy.
Keywords: calcium overload; immunotherapy; radiodynamic therapy; second messengers; smart nanotherapeutic.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Oe Y., Wang X., Patriarchi T., Konno A., Ozawa K., Yahagi K., Hirai H., Tsuboi T., Kitaguchi T., Tian L., Nat. Commun. 2020, 11, 471; - PMC - PubMed
- b) Slavik K. M., Morehouse B. R., Ragucci A. E., Zhou W., Ai X., Chen Y., Li L., Wei Z., Bähre H., König M., Nature 2021, 597, 109; - PMC - PubMed
- c) Radhakrishnan A., Rohatgi R., Siebold C., Nat. Chem. Biol. 2020, 16, 1303. - PMC - PubMed
-
- a) Zheng P., Ding J., Asian J. Pharm. Sci. 2022, 17, 1; - PMC - PubMed
- b) Cui C., Merritt R., Fu L., Pan Z., Acta Pharm. Sin. B 2017, 7, 3; - PMC - PubMed
- c) Prise K. M., O'sullivan J. M., Nat. Rev. Cancer 2009, 9, 351; - PMC - PubMed
- d) Kang Y., Xu L., Dong J., Huang Y., Yuan X., Li R., Chen L., Wang Z., Ji X., Coord. Chem. Rev. 2023, 481, 215050.
-
- a) Xu L., Tong G., Song Q., Zhu C., Zhang H., Shi J., Zhang Z., ACS Nano 2018, 12, 6806; - PubMed
- b) Dong Z., Feng L., Hao Y., Li Q., Chen M., Yang Z., Zhao H., Liu Z., Chem 2020, 6, 1391;
- c) Zheng P., Ding B., Shi R., Jiang Z., Xu W., Li G., Ding J., Chen X., Adv. Mater. 2021, 33, 2007426. - PubMed
-
- a) Shae D., Becker K. W., Christov P., Yun D. S., Lytton‐Jean A. K., Sevimli S., Ascano M., Kelley M., Johnson D. B., Balko J. M., Nat. Nanotechnol. 2019, 14, 269; - PMC - PubMed
- b) Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z. J., Science 2013, 339, 826; - PMC - PubMed
- c) Chin E. N., Yu C., Vartabedian V. F., Jia Y., Kumar M., Gamo A. M., Vernier W., Ali S. H., Kissai M., Lazar D. C., Science 2020, 369, 993; - PubMed
- d) Zhan M., Yu X., Zhao W., Peng Y., Peng S., Li J., Lu L., J. Nanobiotechnol. 2022, 20, 23. - PMC - PubMed
-
- a) Koshy S. T., Cheung A. S., Gu L., Graveline A. R., Mooney D. J., Adv. Biosyst. 2017, 1, 1600013; - PMC - PubMed
- b) Chattopadhyay S., Liu Y.‐H., Fang Z.‐S., Lin C.‐L., Lin J.‐C., Yao B.‐Y., Hu C.‐M. J., Nano Lett. 2020, 20, 2246; - PubMed
- c) Gong W., Zhao T., Yuan H., Yang G., Wang W., Li X., Ju H., Nano Today 2024, 58, 102465.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
