Identification of the Polo-like kinase substrate required for homologous synapsis
- PMID: 39680026
- PMCID: PMC11648704
- DOI: 10.1083/jcb.202408092
Identification of the Polo-like kinase substrate required for homologous synapsis
Abstract
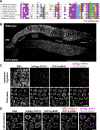
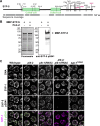
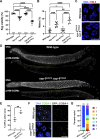
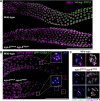
The synaptonemal complex (SC) is a zipper-like protein structure that aligns homologous chromosome pairs and regulates recombination during meiosis. Despite its conserved appearance and function, how synapsis occurs between chromosome axes remains elusive. Here, we demonstrate that Polo-like kinases (PLKs) phosphorylate a single conserved residue in the disordered C-terminal tails of two paralogous SC subunits, SYP-5 and SYP-6, to establish an electrostatic interface between the SC central region and chromosome axes in C. elegans. While SYP-5/6 phosphorylation is dispensable for the ability of SC proteins to self-assemble, local phosphorylation by PLKs at the pairing center is crucial for SC elongation between homologous chromosome axes. Additionally, SYP-5/6 phosphorylation is essential for asymmetric SC disassembly and proper PLK-2 localization after crossover designation, which drives chromosome remodeling required for homolog separation during meiosis I. This work identifies a key regulatory mechanism by which localized PLK activity mediates the SC-axis interaction through phosphorylation of SYP-5/6, coupling synapsis initiation to homolog pairing.
© 2024 Gold et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





Update of
-
Identification of the Polo-like kinase substrate required for homologous synapsis in C. elegans.bioRxiv [Preprint]. 2024 Aug 13:2024.08.13.607834. doi: 10.1101/2024.08.13.607834. bioRxiv. 2024. Update in: J Cell Biol. 2025 Mar 3;224(3):e202408092. doi: 10.1083/jcb.202408092. PMID: 39211260 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

