A spatial threshold for astrocyte calcium surge
- PMID: 39680037
- PMCID: PMC11649242
- DOI: 10.7554/eLife.90046
A spatial threshold for astrocyte calcium surge
Abstract
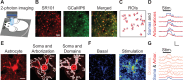
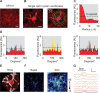
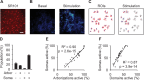
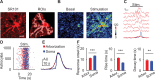
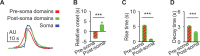
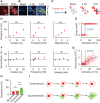
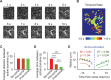
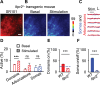
Astrocytes are active cells involved in brain function through the bidirectional communication with neurons, in which astrocyte calcium plays a crucial role. Synaptically evoked calcium increases can be localized to independent subcellular domains or expand to the entire cell, i.e., calcium surge. Because a single astrocyte may contact ~100,000 synapses, the control of the intracellular calcium signal propagation may have relevant consequences on brain function. Yet, the properties governing the spatial dynamics of astrocyte calcium remains poorly defined. Imaging subcellular responses of cortical astrocytes to sensory stimulation in mice, we show that sensory-evoked astrocyte calcium responses originated and remained localized in domains of the astrocytic arborization, but eventually propagated to the entire cell if a spatial threshold of >23% of the arborization being activated was surpassed. Using Itpr2-/- mice, we found that type-2 IP3 receptors were necessary for the generation of astrocyte calcium surge. We finally show using in situ electrophysiological recordings that the spatial threshold of the astrocyte calcium signal consequently determined the gliotransmitter release. Present results reveal a fundamental property of astrocyte physiology, i.e., a spatial threshold for astrocyte calcium propagation, which depends on astrocyte intrinsic properties and governs astrocyte integration of local synaptic activity and subsequent neuromodulation.
Keywords: astrocyte; calcium; cell biology; integration; mouse; neuroscience; synaptic integration.
© 2023, Lines et al.
Conflict of interest statement
JL, AB, CN, EM, JA, PK, MN, AA No competing interests declared
Figures

Update of
-
A spatial threshold for astrocyte calcium surge.bioRxiv [Preprint]. 2024 Aug 8:2023.07.18.549563. doi: 10.1101/2023.07.18.549563. bioRxiv. 2024. Update in: Elife. 2024 Dec 16;12:RP90046. doi: 10.7554/eLife.90046. PMID: 37503130 Free PMC article. Updated. Preprint.
References
-
- Ahmadpour N, Kantroo M, Stobart MJ, Meza-Resillas J, Shabanipour S, Parra-Nuñez J, Salamovska T, Muzaleva A, O’Hara F, Erickson D, Di Gaetano B, Carrion-Falgarona S, Weber B, Lamont A, Lavine NE, Kauppinen TM, Jackson MF, Stobart JL. Cortical astrocyte N-methyl-D-aspartate receptors influence whisker barrel activity and sensory discrimination in mice. Nature Communications. 2024;15:1571. doi: 10.1038/s41467-024-45989-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- K99 AG084853/AG/NIA NIH HHS/United States
- BFU2017-88393-P/Ministerio de Ciencia, Innovación y Universidades
- F31 AG057155/AG/NIA NIH HHS/United States
- R01MH119355/MH/NIMH NIH HHS/United States
- PRX19/00646/Salvador de Madariaga Program
- R01 DA048822/DA/NIDA NIH HHS/United States
- BA15/00078/Instituto de Salud Carlos III
- PID2019-105020GB-100/Ministerio de Ciencia e Innovación
- RTI2018-094887-B-I00/Ministerio de Ciencia e Innovación
- W911NF2110328/Department of Defense Education Activity
- F31AG057155/AG/NIA NIH HHS/United States
- Doctoral Dissertation Fellowship/University of Minnesota
- R01 MH119355/MH/NIMH NIH HHS/United States
- PID2021-122586NB-I00/Ministerio de Ciencia e Innovación
- R01DA048822/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

