Giant axonal neuropathy (GAN): cross-sectional data on phenotypes, genotypes, and proteomic signature from a German cohort
- PMID: 39680150
- PMCID: PMC11649756
- DOI: 10.1007/s00415-024-12744-z
Giant axonal neuropathy (GAN): cross-sectional data on phenotypes, genotypes, and proteomic signature from a German cohort
Abstract
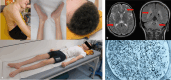
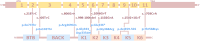
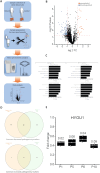
Giant axonal neuropathy (GAN) is a progressive neurodegenerative disease affecting the peripheral and central nervous system and is caused by bi-allelic variants in the GAN gene, leading to loss of functional gigaxonin protein. A treatment does not exist, but a first clinical trial using a gene therapy approach has recently been completed. Here, we conducted the first systematic study of GAN patients treated by German-speaking child neurologists. We collected clinical, genetic, and epidemiological data from a total of 15 patients representing one of the largest cohorts described thus far. Average age of patients was 11.7 years at inclusion. The most frequently reported symptoms (HPO coded) were gait disturbance and muscle weakness, abnormality of muscle size, and abnormal reflexes. In line with the frequency of homozygous variants, in five families, parents reported being at least distantly related. In 14 patients, diagnosis was confirmed by molecular genetic testing, revealing eight different GAN variants, four being reported as pathogenic in the literature. Proteomics of white blood cells derived from four patients was conducted to obtain unbiased insights into the underlying pathophysiology and revealed dysregulation of 111 proteins implicated in diverse biological processes. Of note, diverse of these proteins is known to be crucial for proper synaptic function and transmission and affection of intermediate filament organisation and proteolysis, which is in line with the known functions of gigaxonin.
Keywords: Consanguinity; GAN Gene; Giant axonal neuropathy; Gigaxonin; Neurodegenrative; Proteomics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: The authors declare that they have no conflict of interest. Reviewer information: Reviewer can access the proteomic dataset by logging in to the PRIDE website using the following account details: Username: reviewer_pxd053070@ebi.ac.uk. Password: qVhgNbq4WUBg.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

