Lithium Use During Pregnancy in 14 Countries
- PMID: 39680408
- PMCID: PMC11650410
- DOI: 10.1001/jamanetworkopen.2024.51117
Lithium Use During Pregnancy in 14 Countries
Abstract
Importance: In pregnancy, the benefits of lithium treatment for relapse prevention in psychiatric conditions must be weighed against potential teratogenic effects. Currently, there is a paucity of information on how and when lithium is used by pregnant women.
Objective: To examine lithium use in the perinatal period.
Design, setting, and participants: This cohort study used individual-level data of pregnancies from January 1, 2000, to December 31, 2021, in Australia, Denmark, Finland, Germany, Hong Kong, Iceland, Israel, New Zealand, Norway, South Korea, Sweden, Taiwan, the UK, and 2 cohorts in the US. Analyses were performed from September 1 to November 30, 2023.
Exposures: The prevalence of lithium use as the proportion of pregnancies with at least 1 prescription fill or prescription within 3 months before pregnancy until childbirth was estimated using a common protocol. Lithium use during pregnancy by trimester and in the 3 months before and after pregnancy was examined.
Main outcomes and measures: Comparison of prevalence between the first and last 3-year periods of available data.
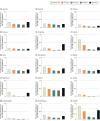
Results: Among 21 659 454 pregnancies from all collaborating sites, the prevalence of lithium use ranged from 0.07 per 1000 pregnancies in Hong Kong to 1.56 per 1000 in the US publicly insured population. Lithium use increased per 1000 pregnancies in 10 populations (Australia [0.60 to 0.74], Denmark [0.09 to 0.51], Finland [0.10 to 0.29], Iceland [0.24 to 0.99], Israel [0.25 to 0.37], Norway [0.24 to 0.47], South Korea [0.30 to 0.44], Sweden [0.42 to 1.07], the UK [0.07 to 0.10], and Taiwan [0.15 to 0.19]), remained stable in 4 populations (Germany [0.17 to 0.16], Hong Kong [0.06 to 0.06], and the publicly [1.50 to 1.34] and commercially [0.38 to 0.36] insured US populations), and decreased in 1 population (New Zealand [0.54 to 0.39]). Use of lithium decreased with each trimester of pregnancy, while prevalence of postpartum use was similar to prepregnancy levels. The proportion of lithium use in the second trimester compared with the prepregnancy period ranged from 2% in South Korea to 80% in Denmark.
Conclusions and relevance: Prevalence of lithium use in pregnant women over the past 2 decades varied markedly between populations. Patterns of use before, during, and after pregnancy suggest that many women discontinued lithium use during pregnancy and reinitiated treatment after childbirth, with large variations between countries. These findings underscore the need for internationally harmonized guidelines, specifically for psychiatric conditions among pregnant women that may benefit from lithium treatment.
Conflict of interest statement
Figures

References
-
- Goodwin FK, Jamison KR, Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd ed. Oxford University Press; 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

