US FDA Approval of Pediatric Artificial Intelligence and Machine Learning-Enabled Medical Devices
- PMID: 39680415
- PMCID: PMC11791695
- DOI: 10.1001/jamapediatrics.2024.5437
US FDA Approval of Pediatric Artificial Intelligence and Machine Learning-Enabled Medical Devices
Plain language summary
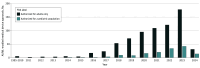
This cross-sectional study analyzes the availability of artificial intelligence and machine learning–enabled devices authorized for children by the US Food and Drug Administration (FDA) and assesses reporting of algorithm validation in the pediatric population.
Conflict of interest statement
Figures
References
-
- US Food and Drug Administration . Marketing submission recommendations for a predetermined change control plan for artificial intelligence/machine learning (AI/ML)-enabled device software functions: draft guidance for industry and Food and Drug Administration staff. Accessed June 1, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- US Food and Drug Administration . Artificial intelligence and machine learning (AI/ML)-enabled medical devices. Accessed March 31, 2024. https://www.fda.gov/medical-devices/software-medical-device-samd/artific...
LinkOut - more resources
Full Text Sources