Unsupervised discovery of family specific vocal usage in the Mongolian gerbil
- PMID: 39680425
- PMCID: PMC11649239
- DOI: 10.7554/eLife.89892
Unsupervised discovery of family specific vocal usage in the Mongolian gerbil
Abstract
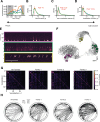
In nature, animal vocalizations can provide crucial information about identity, including kinship and hierarchy. However, lab-based vocal behavior is typically studied during brief interactions between animals with no prior social relationship, and under environmental conditions with limited ethological relevance. Here, we address this gap by establishing long-term acoustic recordings from Mongolian gerbil families, a core social group that uses an array of sonic and ultrasonic vocalizations. Three separate gerbil families were transferred to an enlarged environment and continuous 20-day audio recordings were obtained. Using a variational autoencoder (VAE) to quantify 583,237 vocalizations, we show that gerbils exhibit a more elaborate vocal repertoire than has been previously reported and that vocal repertoire usage differs significantly by family. By performing gaussian mixture model clustering on the VAE latent space, we show that families preferentially use characteristic sets of vocal clusters and that these usage preferences remain stable over weeks. Furthermore, gerbils displayed family-specific transitions between vocal clusters. Since gerbils live naturally as extended families in complex underground burrows that are adjacent to other families, these results suggest the presence of a vocal dialect which could be exploited by animals to represent kinship. These findings position the Mongolian gerbil as a compelling animal model to study the neural basis of vocal communication and demonstrates the potential for using unsupervised machine learning with uninterrupted acoustic recordings to gain insights into naturalistic animal behavior.
Keywords: auditory neuroscience; bioacoustics; ecology; ethology; mongolian gerbil; mongolian gerbil (meriones unguiculatus); neuroscience; social behavior; vocal communication.
Plain language summary
Every time you speak, the sounds coming out of your mouth may carry more meaning that you may have intended; they may reveal, for example, which country, city or even neighborhood you may be coming from. Indeed, the vocal patterns that humans use to communicate differ from one population to the next, creating an array of languages, dialects and accents. Such diversity has also been identified in various social species across the animal kingdom. Naked mole rats, for instance, which live underground in complex societies, exhibit different ‘dialects’ depending on their group of origin. Yet studying the vocal patterns of animals has remained difficult, especially for species inhabiting burrows or other environments difficult to access. Aiming to bypass these limitations, Peterson et al. adopted a ‘naturalistic’ approach that allowed them to capture the vocal calls of three families of Mongolian gerbils living undisturbed in enclosures that mimic features of their natural environment. These animals spend their lives underground in tight-knit families, with multiple groups often being in close proximity. Researchers have speculated that individuals may rely on vocal cues to identify whether they are part of the same colony, as they are often too far from each other to rely on sight or smell. Over half a million vocalizations obtained continuously through the course of 20 days were analyzed using an artificial intelligence technique known as unsupervised machine learning. The analyses helped add new types of calls to the gerbil vocal repertoire, but also highlighted its complexity. In particular, they revealed that the animals could combine individual vocal elements into complex sequences. More importantly, this approach showed that gerbil families have vocal dialects that are stable across weeks, with each group displaying a preference for certain call types (i.e. words) and certain sequential patterns (i.e. phrases). These findings demonstrate the benefits of the approach developed by Peterson et al. for the study of animal vocalizations. Going forward, they also suggest that the Mongolian gerbil could be used as an animal model to study the neural basis of vocal communication.
© 2023, Peterson et al.
Conflict of interest statement
RP, AC, CM, AT, AC, AW, DS, DS No competing interests declared
Figures






Update of
-
Unsupervised discovery of family specific vocal usage in the Mongolian gerbil.bioRxiv [Preprint]. 2024 Sep 4:2023.03.11.532197. doi: 10.1101/2023.03.11.532197. bioRxiv. 2024. Update in: Elife. 2024 Dec 16;12:RP89892. doi: 10.7554/eLife.89892. PMID: 39282260 Free PMC article. Updated. Preprint.
References
-
- Ågren G. Pair formation in the Mongolian gerbil. Animal Behaviour. 1984a;32:528–535. doi: 10.1016/S0003-3472(84)80291-2. - DOI
-
- Ågren G. Incest avoidance and bonding between siblings in gerbils. Behavioral Ecology and Sociobiology. 1984b;14:161–169. doi: 10.1007/BF00299615. - DOI
-
- Ågren G, Zhou Q, Zhong W. Ecology and social behaviour of Mongolian gerbils,Meriones unguiculatus, at Xilinhot, Inner Mongolia, China. Animal Behaviour. 1989a;37:11–27. doi: 10.1016/0003-3472(89)90002-X. - DOI
-
- Ågren G, Zhou Q, Zhong W. Territoriality, cooperation and resource priority: hoarding in the Mongolian gerbil,Meriones unguiculatus. Animal Behaviour. 1989b;37:28–32. doi: 10.1016/0003-3472(89)90003-1. - DOI
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

