Contributions of associative and non-associative learning to the dynamics of defensive ethograms
- PMID: 39680437
- PMCID: PMC11649235
- DOI: 10.7554/eLife.90414
Contributions of associative and non-associative learning to the dynamics of defensive ethograms
Abstract
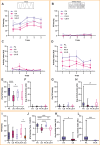
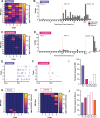
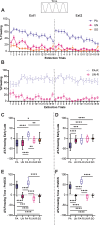
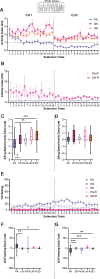
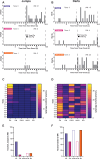
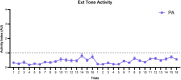
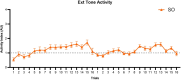
Defensive behavior changes based on threat intensity, proximity, and context of exposure, and learning about danger-predicting stimuli is critical for survival. However, most Pavlovian fear conditioning paradigms focus only on freezing behavior, obscuring the contributions of associative and non-associative mechanisms to dynamic defensive responses. To thoroughly investigate defensive ethograms, we subjected male and female adult C57BL/6 J mice to a Pavlovian conditioning paradigm that paired footshock with a serial compound stimulus (SCS) consisting of distinct tone and white noise (WN) stimulus periods. To investigate how associative and non-associative mechanisms affect defensive responses, we compared this paired SCS-footshock group with four control groups that were conditioned with either pseudorandom unpaired presentations of SCS and footshock, shock only, or reversed SCS presentations with inverted tone-WN order, with paired or unpaired presentations. On day 2 of conditioning, the paired group exhibited robust freezing during the tone period with switching to explosive jumping and darting behaviors during the WN period. Comparatively, the unpaired and both reverse SCS groups expressed less tone-induced freezing and rarely showed jumping or darting during WN. Following the second day of conditioning, we observed how defensive behavior changed over two extinction sessions. During extinction, the tone-induced freezing decreased in the paired group, and mice rapidly shifted from escape jumping during WN to a combination of freezing and darting. The unpaired, unpaired reverse, and shock-only groups displayed defensive tail rattling and darting during the SCS, with minimal freezing and jumping. Interestingly, the paired reverse group did not jump to WN, and tone-evoked freezing was resistant to extinction. These findings demonstrate that non-associative factors promote some defensive responsiveness, but associative factors are required for robust cue-induced freezing and high-intensity flight expression.
Keywords: associative learning; defensive behavior; fear; fear extinction; mouse; neuroscience; non-associative learning; pavlovian conditioning.
Plain language summary
Post-traumatic stress disorder (or PTSD for short) is a condition that can cause people to overreact to harmless cues, vividly re-experience a traumatic event, or freeze in place. To understand why this happens, researchers often study fear responses using an approach called fear conditioning, where laboratory animals learn to associate the sound of a tone with a mild electric shock. This conditioning causes animals to freeze with fear when they hear the tone. However, focusing on freezing overlooks the range of defensive actions animals may carry out, such as escaping or fighting. Capturing this complexity in experiments is important for understanding the dynamic nature of fear responses that occur in PTSD. Previous work showed that conditioning mice with a two-part cue, such as a tone followed by white noise, caused mice to freeze during the first cue and jump during the second cue. However, whether the mice learned this behaviour through conditioning or if it was an instinctive response to the cues remained unclear. To investigate this phenomenon, Le et al. – including some of the researchers involved in the previous work – conditioned mice with a variety of different cue combinations and monitored how they responded. As before, mice conditioned to associate a tone followed by white noise with an electric shock froze when they heard the tone and transitioned to jumping during the white noise. However, if during conditioning the sounds and shocks occurred at unpredictable times, the mice did not associate the sounds with the shock and therefore they froze less and rarely jumped. Similarly, reversing the order of the sounds so that the white noise happened before the tone also reduced jumping but not freezing. To investigate whether the mice could unlearn this fear response, Le et al. exposed the fear-conditioned mice to the cues without an accompanying electric shock. The mice that had been conditioned with a tone followed by white noise showed a weaker response to the cues, only freezing and not jumping. However, the mice with the reversed cues still froze even after this exposure, and the mice with the non-associated cues maintained very little freezing and jumping. Taken together, the findings suggest that while fear responses can be influenced by the association between certain noises and an electric shock, other factors such as the timing and the order of the sound cues can also impact the intensity of the fear response. The experiments also showed that this method of fear conditioning can be used for both learning and unlearning fear responses, revealing an approach for future studies into how fear responses change over time. Combining this more complex approach with other experimental techniques could help researchers identify the brain regions that drive fear responses, which may eventually benefit people with PTSD and other fear disorders.
© 2023, Le et al.
Conflict of interest statement
QL, DH, CB, ZA, JK, AR, JF No competing interests declared
Figures




Update of
-
Contributions of associative and non-associative learning to the dynamics of defensive ethograms.bioRxiv [Preprint]. 2024 Sep 19:2023.07.06.547975. doi: 10.1101/2023.07.06.547975. bioRxiv. 2024. Update in: Elife. 2024 Dec 16;12:RP90414. doi: 10.7554/eLife.90414. PMID: 39345429 Free PMC article. Updated. Preprint.
References
-
- Akmese C, Sevinc C, Halim S, Unal G. Differential role of GABAergic and cholinergic ventral pallidal neurons in behavioral despair, conditioned fear memory and active coping. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2023;125:110760. doi: 10.1016/j.pnpbp.2023.110760. - DOI - PubMed
-
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychological Review. 1970;77:32–48. doi: 10.1037/h0028589. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

