SGLT2 inhibition alters substrate utilization and mitochondrial redox in healthy and failing rat hearts
- PMID: 39680452
- PMCID: PMC11645152
- DOI: 10.1172/JCI176708
SGLT2 inhibition alters substrate utilization and mitochondrial redox in healthy and failing rat hearts
Abstract
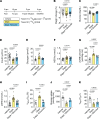
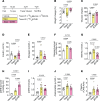
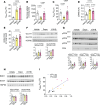
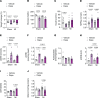
Previous studies highlight the potential for sodium-glucose cotransporter type 2 (SGLT2) inhibitors (SGLT2i) to exert cardioprotective effects in heart failure by increasing plasma ketones and shifting myocardial fuel utilization toward ketone oxidation. However, SGLT2i have multiple in vivo effects and the differential impact of SGLT2i treatment and ketone supplementation on cardiac metabolism remains unclear. Here, using gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodology combined with infusions of [13C6]glucose or [13C4]βOHB, we demonstrate that acute SGLT2 inhibition with dapagliflozin shifts relative rates of myocardial mitochondrial metabolism toward ketone oxidation, decreasing pyruvate oxidation with little effect on fatty acid oxidation in awake rats. Shifts in myocardial ketone oxidation persisted when plasma glucose levels were maintained. In contrast, acute βOHB infusion similarly augmented ketone oxidation, but markedly reduced fatty acid oxidation and did not alter glucose uptake or pyruvate oxidation. After inducing heart failure, dapagliflozin increased relative rates of ketone and fatty acid oxidation, but decreased pyruvate oxidation. Dapagliflozin increased mitochondrial redox and reduced myocardial oxidative stress in heart failure, which was associated with improvements in left ventricular ejection fraction after 3 weeks of treatment. Thus, SGLT2i have pleiotropic effects on systemic and heart metabolism, which are distinct from ketone supplementation and may contribute to the long-term cardioprotective benefits of SGLT2i.
Keywords: Cardiology; Glucose metabolism; Intermediary metabolism; Metabolism; Mitochondria.
Conflict of interest statement
Figures




Comment in
- Beyond ketosis: the search for the mechanism underlying SGLT2-inhibitor benefit continues doi: 10.1172/JCI187097
References
-
- Cosentino F, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142(23):2205–2215. doi: 10.1161/CIRCULATIONAHA.120.050255. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

