(Re)building the nervous system: A review of neuron-glia interactions from development to disease
- PMID: 39680483
- PMCID: PMC11649038
- DOI: 10.1111/jnc.16258
(Re)building the nervous system: A review of neuron-glia interactions from development to disease
Abstract
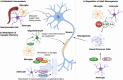
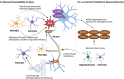
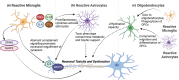
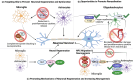
Neuron-glia interactions are fundamental to the development and function of the nervous system. During development, glia, including astrocytes, microglia, and oligodendrocytes, influence neuronal differentiation and migration, synapse formation and refinement, and myelination. In the mature brain, glia are crucial for maintaining neural homeostasis, modulating synaptic activity, and supporting metabolic functions. Neurons, inherently vulnerable to various stressors, rely on glia for protection and repair. However, glia, in their reactive state, can also promote neuronal damage, which contributes to neurodegenerative and neuropsychiatric diseases. Understanding the dual role of glia-as both protectors and potential aggressors-sheds light on their complex contributions to disease etiology and pathology. By appropriately modulating glial activity, it may be possible to mitigate neurodegeneration and restore neuronal function. In this review, which originated from the International Society for Neurochemistry (ISN) Advanced School in 2019 held in Montreal, Canada, we first describe the critical importance of glia in the development and maintenance of a healthy nervous system as well as their contributions to neuronal damage and neurological disorders. We then discuss potential strategies to modulate glial activity during disease to protect and promote a properly functioning nervous system. We propose that targeting glial cells presents a promising therapeutic avenue for rebuilding the nervous system.
Keywords: astrocyte; brain development and function; glial dysfunction; microglia; neurodegenerative and neuropsychiatric disorders; oligodendrocyte.
© 2024 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Abbott, N. J. , Rönnbäck, L. , & Hansson, E. (2006). Astrocyte‐endothelial interactions at the blood‐brain barrier. Nature Reviews. Neuroscience, 7(1), 41–53. - PubMed
-
- Åberg, M. A. I. , Åberg, N. D. , Palmer, T. D. , Alborn, A. M. , Carlsson‐Skwirut, C. , Bang, P. , Rosengren, L. E. , Olsson, T. , Gage, F. H. , & Eriksson, P. S. (2003). IGF‐I has a direct proliferative effect in adult hippocampal progenitor cells. Molecular and Cellular Neuroscience, 24, 23–40. - PubMed
-
- Albrecht, P. J. , Murtie, J. C. , Ness, J. K. , Redwine, J. M. , Enterline, J. R. , Armstrong, R. C. , & Levison, S. W. (2003). Astrocytes produce CNTF during the remyelination phase of viral‐induced spinal cord demyelination to stimulate FGF‐2 production. Neurobiology of Disease, 13, 89–101. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

