Perihilar Cholangiocarcinoma Originating in Peribiliary Glands: Insights from a Case without Precancerous Lesions
- PMID: 39680512
- PMCID: PMC11660006
- DOI: 10.12659/AJCR.945519
Perihilar Cholangiocarcinoma Originating in Peribiliary Glands: Insights from a Case without Precancerous Lesions
Abstract
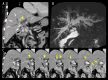
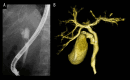
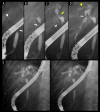
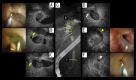
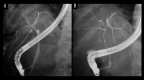
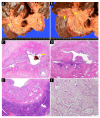
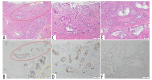
BACKGROUND Recent studies have shown that peribiliary glands may be the potential cell origin of cholangiocarcinoma, and that precancerous lesions such as biliary intraepithelial neoplasms and intraductal papillary neoplasms of the bile duct may arise from these peribiliary glands. However, whether and how these precancerous lesions progress to cholangiocarcinoma is controversial. CASE REPORT Herein, an autopsy case of perihilar cholangiocarcinoma, exclusively periductal-infiltrating, is reported. Since repeated transpapillary biopsies and cytology showed no carcinoma cells, the patient was treated for sclerosing cholangitis until death. The findings at cholelithiasis treatment 1 year earlier had not aroused suspicion of the presence of precancerous lesions. The changes in the spread of bile duct stenoses on cholangiography and the unique findings at autopsy, namely (i) the distribution of cancer growing locally within the peribiliary gland compartment without invading the bile duct mucosa and (ii) the existence of in situ-like carcinoma cells replacing the epithelium of the peribiliary glands throughout the extrahepatic bile duct, suggested that cholangiocarcinoma arose from the peribiliary glands in the hilum without a detectable precancerous lesion and then spread to the lower end of the common bile duct via the peribiliary gland network. CONCLUSIONS This case report may help further our understanding of the natural history of cholangiocarcinoma and provide clues about cholangiocarcinogenesis and progression. In addition, histological and cytological diagnosis could be theoretically difficult by sampling tissue from the bile duct lumen in cholangiocarcinoma, as in this case.
Conflict of interest statement
Figures

References
-
- Lanzoni G, Cardinale V, Carpino G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology. 2016;64(1):277–86. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

