Dipeptidyl Peptidase 4 (DPP4) Exacerbates Osteoarthritis Progression in an Enzyme-Independent Manner
- PMID: 39680708
- PMCID: PMC11809337
- DOI: 10.1002/advs.202410525
Dipeptidyl Peptidase 4 (DPP4) Exacerbates Osteoarthritis Progression in an Enzyme-Independent Manner
Abstract
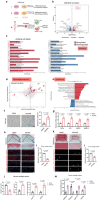
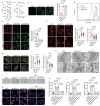
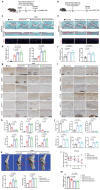
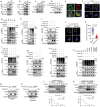
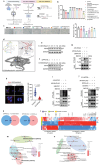
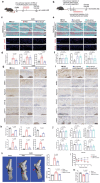
Chondrocyte senescence is a key driver of osteoarthritis (OA). Mitochondrial dysfunction and oxidative stress can induce chondrocyte senescence. However, the specific mechanisms by which senescence contributes to OA progression are not fully understood. Here, it is attested that Dipeptidyl peptidase 4 (DPP4) is significantly upregulated in osteoarthritic chondrocytes in both humans and mice. DPP4 promotes oxidative stress and cellular senescence in chondrocytes through excessive mitochondrial fission in an enzyme-independent manner. Intra-articular injection of adeno-associated virus 2 to upregulate DPP4 in chondrocytes promotes post-traumatic and aging-induced OA in mice in an enzyme-independent manner. Mechanistically, DPP4 competitively binds to Myosin heavy chain 9 (MYH9), interfering with its E3 ubiquitin ligase Carboxyl terminus of Hsc70-interacting protein (CHIP), and thereby upregulates MYH9 expression. Finally, a small molecule, 4,5-Dicaffeoylquinic acid is identified, which disrupts the interaction between DPP4 and MYH9, thereby ameliorating post-traumatic and aging-induced OA in mice caused by DPP4 upregulation. The study indicates that the non-enzymatic activity of DPP4 is a promising target for OA treatment.
Keywords: DPP4; MYH9; mitochondrial dynamics; osteoarthritis; senescence.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- G. B. D. O. Collaborators , Lancet Rheumatol. 2023, 5, e508. - PubMed
-
- Hunter D. J., Bierma‐Zeinstra S., Lancet 2019, 393, 1745. - PubMed
-
- Kolasinski S. L., Neogi T., Hochberg M. C., Oatis C., Guyatt G., Block J., Callahan L., Copenhaver C., Dodge C., Felson D., Gellar K., Harvey W. F., Hawker G., Herzig E., Kwoh C. K., Nelson A. E., Samuels J., Scanzello C., White D., Wise B., Altman R. D., DiRenzo D., Fontanarosa J., Giradi G., Ishimori M., Misra D., Shah A. A., Shmagel A. K., Thoma L. M., Turgunbaev M., et al., Arthritis Care Res. (Hoboken) 2020, 72, 149. - PMC - PubMed
-
- a) Cao H., Yang P., Liu J., Shao Y., Li H., Lai P., Wang H., Liu A., Guo B., Tang Y., Bai X., Li K., Nat. Commun. 2023, 14, 6190; - PMC - PubMed
- b) Loeser R. F., Kelley K. L., Armstrong A., Collins J. A., Diekman B. O., Carlson C. S., Arthritis Rheumatol. 2020, 72, 1679; - PMC - PubMed
- c) Collins J. A., Kim C. J., Coleman A., Little A., Perez M. M., Clarke E. J., Diekman B., Peffers M. J., Chubinskaya S., Tomlinson R. E., Freeman T. A., Loeser R. F., Ann. Rheum. Dis. 2023, 82, 1464. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
