Biofilm formation by Staphylococcus aureus is triggered by a drop in the levels of a cyclic dinucleotide
- PMID: 39680756
- PMCID: PMC11670122
- DOI: 10.1073/pnas.2417323121
Biofilm formation by Staphylococcus aureus is triggered by a drop in the levels of a cyclic dinucleotide
Abstract
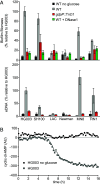
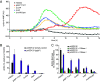
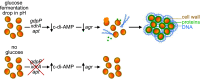
The bacterial pathogen Staphylococcus aureus forms multicellular communities known as biofilms in which cells are held together by an extracellular matrix principally composed of repurposed cytoplasmic proteins and extracellular DNA. These biofilms assemble during infections or under laboratory conditions by growth on medium containing glucose, but the intracellular signal for biofilm formation and its downstream targets were unknown. Here, we present evidence that biofilm formation is triggered by a drop in the levels of the second messenger cyclic-di-AMP. Previous work identified genes needed for the release of extracellular DNA, including genes for the cyclic-di-AMP phosphodiesterase GdpP, the transcriptional regulator XdrA, and the purine salvage enzyme Apt. Using a cyclic-di-AMP riboswitch biosensor and mass spectrometry, we show that the second messenger drops in abundance during biofilm formation in a glucose-dependent manner. Mutation of these three genes elevates cyclic-di-AMP and prevents biofilm formation in a murine catheter model. Supporting the generality of this mechanism, we found that gdpP was required for biofilm formation by diverse strains of S. aureus. We additionally show that the downstream consequence of the drop in cyclic-di-AMP is inhibition of the "accessory gene regulator" operon agr, which is known to suppress biofilm formation through phosphorylation of the transcriptional regulator AgrA by the histidine kinase AgrC. Consistent with this, an agr mutation bypasses the block in biofilm formation and eDNA release caused by a gdpP mutation. Finally, we report the unexpected observation that GdpP inhibits phosphotransfer from AgrC to AgrA, revealing a direct connection between the phosphodiesterase and agr.
Keywords: Staphylococcus aureus; biofilm formation; cyclic-di-AMP.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

