The distinctive mechanical and structural signatures of residual force enhancement in myofibers
- PMID: 39680764
- PMCID: PMC11670058
- DOI: 10.1073/pnas.2413883121
The distinctive mechanical and structural signatures of residual force enhancement in myofibers
Abstract
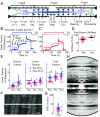
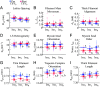
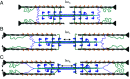
In muscle, titin proteins connect myofilaments together and are thought to be critical for contraction, especially during residual force enhancement (RFE) when steady-state force is elevated after an active stretch. We investigated titin's function during contraction using small-angle X-ray diffraction to track structural changes before and after 50% titin cleavage and in the RFE-deficient, mdm titin mutant. We report that the RFE state is structurally distinct from pure isometric contractions, with increased thick filament strain and decreased lattice spacing, most likely caused by elevated titin-based forces. Furthermore, no RFE structural state was detected in mdm muscle. We posit that decreased lattice spacing, increased thick filament stiffness, and increased non-cross-bridge forces are the major contributors to RFE. We conclude that titin directly contributes to RFE.
Keywords: X-ray diffraction; elasticity; force transmission; mouse; ultrastructure.
Conflict of interest statement
Competing interests statement:T.C.I. provides consulting and collaborative research studies to Edgewise Therapeutics inc. and A.L.H./M.K. are owners of Accelerated Muscle Biotechnologies LLC, but such work is unrelated to the content of this article. All other authors declare that they have no competing interests.
Figures
Update of
-
The distinctive mechanical and structural signatures of residual force enhancement in myofibers.bioRxiv [Preprint]. 2023 Feb 21:2023.02.19.529125. doi: 10.1101/2023.02.19.529125. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Dec 24;121(52):e2413883121. doi: 10.1073/pnas.2413883121. PMID: 36865266 Free PMC article. Updated. Preprint.
References
-
- Dickinson M. H., et al. , How animals move: An integrative view. Science 288, 100–106 (2000). - PubMed
-
- Nishikawa K. C., Muscle function from organisms to molecules. Integr. Comp. Biol. 58, 194–206 (2018). - PubMed
-
- Lindstedt S. L., Skeletal muscle tissue in movement and health: Positives and negatives. J. Exp. Biol. 219, 183–188 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

