Caffeine Therapy for Apnea of Prematurity: Single-Center Study on Dosing Practices and Perceived Effectiveness
- PMID: 39681093
- PMCID: PMC12129425
- DOI: 10.1159/000543074
Caffeine Therapy for Apnea of Prematurity: Single-Center Study on Dosing Practices and Perceived Effectiveness
Abstract
Introduction: Caffeine is the registered pharmacologic treatment for apnea of prematurity and is extensively used in the neonatal intensive care units (NICUs) based on evidence from randomized controlled trials. This study aimed to describe the clinical use of caffeine based on real-world data, hypothesizing a divergence from the registered dosing regimen.
Methods: A retrospective analysis included infants born before 30 weeks of gestation, admitted to the NICU of the Erasmus MC Rotterdam from 2018 to 2021. Exclusion criteria comprised infants admitted after postnatal day 2, those not receiving caffeine during admission, patients admitted for less than 24 h, those who spent less than 24 h on non-invasive support, and cases lacking medication data. The primary outcome was the proportion of patients receiving an average caffeine dose higher than registered on the label.
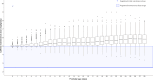
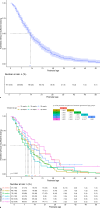
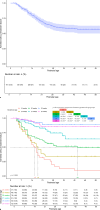
Results: A total of 451 patients with a median gestational age of 28+0 weeks (IQR 26+2-29+0) and birthweight of 1,015 g (IQR 800-1,218) were included. Of these, 402 infants (89%) received an average daily caffeine dosage exceeding the registered dose range. The median caffeine maintenance dose per patient was 5.3 mg/kg/day (IQR 5.0-5.8), with additional therapy (mini-load, doxapram, or intubation) needed in 318 patients (71%).
Conclusion: This study highlights the frequent use of higher caffeine dosages in clinical practice than registered and recommended based on long-term safety data. Despite these high dosages and frequent mini-loads, 28% of patients still required additional treatment with doxapram and/or invasive mechanical ventilation, indicating the need for individualized dosing strategies or alternative therapies.
Keywords: Apnea of prematurity; Caffeine; Neonatal intensive care unit; Pharmacotherapy; Premature infants.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Eichenwald EC; Committee on Fetus and Newborn . Response from committee on fetus and newborn. Pediatrics. 2016;137(5):e20160403B. - PubMed
-
- Martin RJ, Wilson CG. Apnea of prematurity. Compr Physiol. 2012;2(4):2923–31. - PubMed
-
- Rostas SE, McPherson C. Caffeine therapy in preterm infants: the dose (and timing) make the medicine. Neonatal Netw. 2019;38(6):365–74. - PubMed
-
- Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, et al. . Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. 2015;314(6):595–603. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

