Assembling Di- and Polynuclear Cu(I) Complexes with Rigid Thioxanthone-Based Ligands: Structures, Reactivity, and Photoluminescence
- PMID: 39681326
- PMCID: PMC11688670
- DOI: 10.1021/acs.inorgchem.4c03819
Assembling Di- and Polynuclear Cu(I) Complexes with Rigid Thioxanthone-Based Ligands: Structures, Reactivity, and Photoluminescence
Abstract
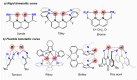
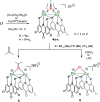
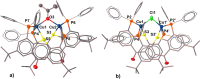
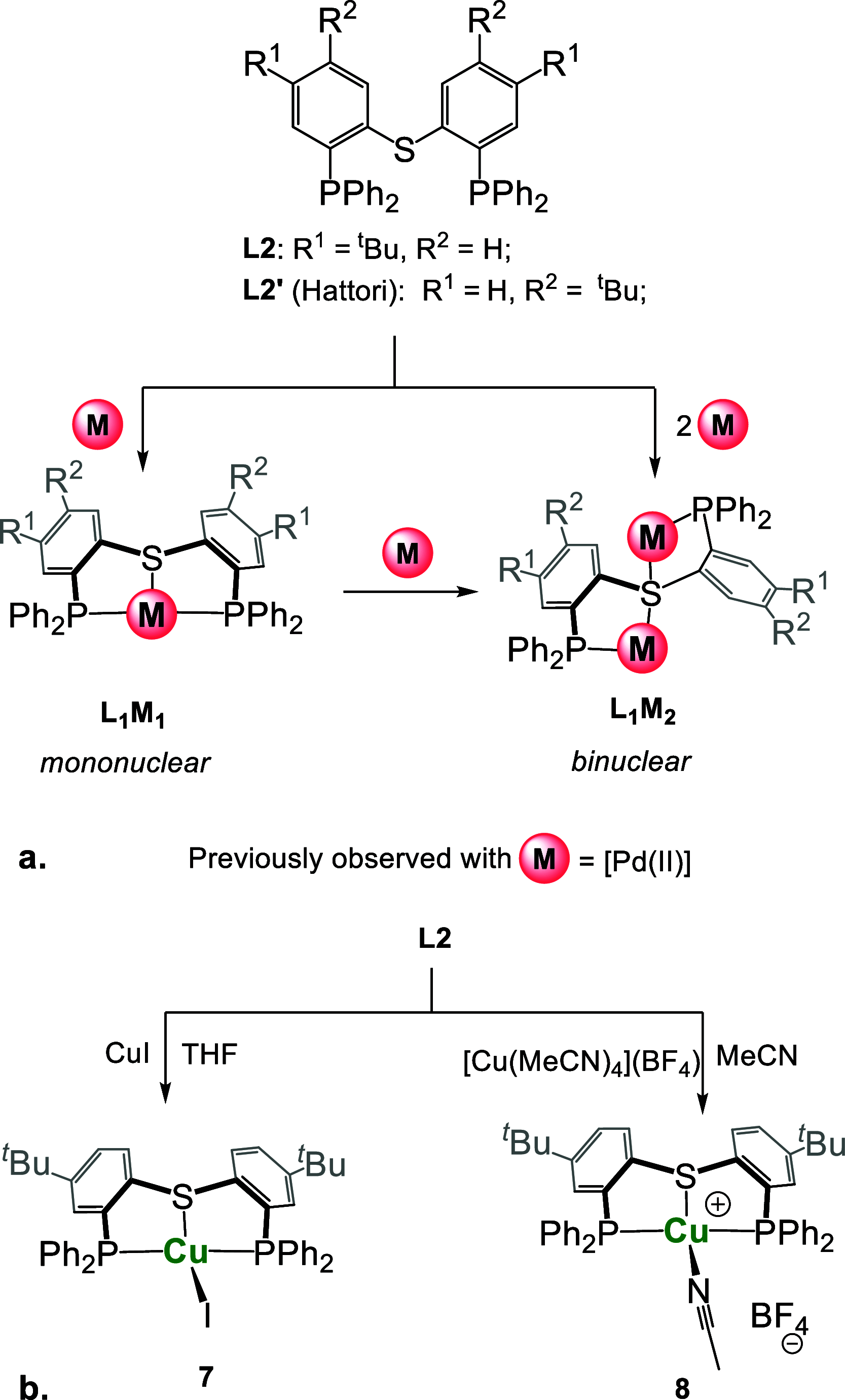
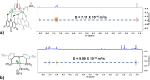
Thioxanthone (TX) molecules and their derivatives are well-known photoactive compounds. Yet, there exist only a handful of luminescent systems combining TX with transition metals. Recently, we reported a TX-based PSP pincer ligand (L1) that appears as a promising platform for filling this niche. Herein, we demonstrate that with Cu(I) this ligand exclusively assembles into dimeric structures with either di- or polynuclear Cu(I) cores. With cationic Cu(I) precursors, complexes featuring solvent-bridged bis-cationic cores were obtained. These coordinatively unsaturated bimetallic systems showed surprisingly facile activation of the chloroform C-Cl bonds, suggesting a possible metal-metal cooperation. The reaction of L1 with binary Cu(I) halides afforded dimeric complexes with polynuclear [CuX]n (n = 3 or 4) cores. With X = Br or I, emissive complexes containing stairstep [CuX]4 clusters were obtained. Emission lifetimes in the microsecond range measured for these complexes were indicative of a triplet emission (phosphorescence), which according to our time-dependent density functional theory study originates from a halide-metal-to-ligand charge transfer between the [CuX]4 cluster and the TX backbone of L1. Finally, the distinctive polynucleating behavior of L1 toward Cu(I) was also showcased by a comparison to another PSP ligand with a diaryl thioether backbone (L2), which formed only mononuclear pincer-type complexes, lacking any unusual reactivity or photoluminescence.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


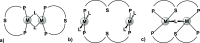


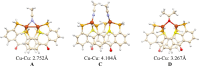

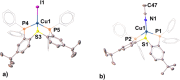


References
-
- van Beek C. B.; van Leest N. P.; Lutz M.; de Vos S. D.; Klein Gebbink R. J. M.; de Bruin B.; Broere D. L. J. Combining metal–metal cooperativity, metal–ligand cooperativity and chemical non-innocence in diiron carbonyl complexes. Chemical Science 2022, 13 (7), 2094–2104. 10.1039/D1SC05473B. - DOI - PMC - PubMed
-
- Farley C. M.; Uyeda C. Organic Reactions Enabled by Catalytically Active Metal–Metal Bonds. Trends in Chemistry 2019, 1 (5), 497–509. 10.1016/j.trechm.2019.04.002. - DOI
-
- Powers I. G.; Uyeda C. Metal–Metal Bonds in Catalysis. ACS Catal. 2017, 7 (2), 936–958. 10.1021/acscatal.6b02692. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

