Clinical impact of cancer cachexia on the outcome of patients with non-small cell lung cancer with PD-L1 tumor proportion scores of ≥50% receiving pembrolizumab monotherapy versus immune checkpoint inhibitor with chemotherapy
- PMID: 39681395
- PMCID: PMC11651275
- DOI: 10.1080/2162402X.2024.2442116
Clinical impact of cancer cachexia on the outcome of patients with non-small cell lung cancer with PD-L1 tumor proportion scores of ≥50% receiving pembrolizumab monotherapy versus immune checkpoint inhibitor with chemotherapy
Abstract
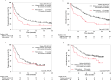
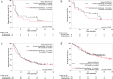
This retrospective, multicenter cohort study aimed to determine whether cancer cachexia serves as a biomarker for determining the most effective treatment for patients having non-small-cell lung cancer (NSCLC) with high programmed death ligand 1 (PD-L1) expression treated with immune checkpoint inhibitors (ICIs) alone or combined with chemotherapy (ICI/chemotherapy). We included 411 patients with advanced NSCLC with a PD-L1 tumor proportion score of ≥50%. The patients were treated with pembrolizumab monotherapy or ICI/chemotherapy. Cancer cachexia was defined as a weight loss of >5% of the total body weight or a body mass index of <20 kg/m2 coupled with an additional weight loss of >2% within 6 months before starting treatment. Eighty-five (21%) patients met the cancer cachexia criteria. Overall survival (OS) was significantly shorter in patients with cachexia than in those without cachexia in both the pembrolizumab monotherapy group (17.2 vs. 35.8 months, p < 0.001) and the ICI/chemotherapy group (27.0 months vs. not reached, p = 0.044). However, after stratifying by cancer cachexia status, no significant difference in OS was observed between the pembrolizumab monotherapy and chemoimmunotherapy groups, regardless of cachexia. In conclusion, ICI/chemotherapy offers limited benefits for NSCLC patients with high PD-L1 expression and concurrent cancer cachexia. Considering the frailty associated with cachexia, ICI monotherapy may be preferred to ICI/chemotherapy for these patients. New interventions that can better address the negative prognostic impact of cachexia in patients treated using ICIs with or without chemotherapy remain warranted.
Keywords: Cancer cachexia; combination therapy; immune checkpoint inhibitor; non-small cell lung cancer; treatment outcome.
Conflict of interest statement
Hayato Kawachi received personal fees from Bristol-Myers Squibb, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan KK, and MSD KK outside the purview of the submitted work. Tadaaki Yamada received research grants from Ono Pharmaceutical, Janssen, AstraZeneca, and Takeda Pharmaceutical and speaking honoraria from Eli Lilly outside the purview of the submitted work. Motohiro Tamiya received research grants from Boehringer Ingelheim, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Daiichi-Sankyo, Eisai, Chugai Pharmaceutical Co. Ltd., and Janssen and personal fees from Chugai Pharmaceutical Co. Ltd., Boehringer Ingelheim, AstraZeneca, Taiho Pharmaceutical, Eli Lilly, Novartis, Pfizer, Asahi Kasei Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Bayer, Amgen, Kyowa-Kirin, and Nippon Kayaku outside the purview of the submitted work. Asuka Okada received personal fees from Chugai-Roshe, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Japan, Nippon Kayaku, and Bristol-Myers Squibb outside the purview of the submitted work. Takashi Kijima received personal fees from Chugai Pharmaceutical Co., Ltd. and MSD KK outside the purview of the submitted work. Koichi Takayama received research grants from Chugai Pharmaceutical Co. Ltd. and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai Pharmaceutical Co. Ltd., MSD-Merck, Eli Lilly, Boehringer Ingelheim, and Daiichi-Sankyo outside the purview of the submitted work.
Figures
References
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
